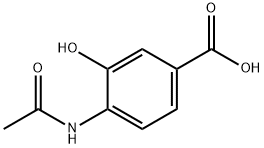
4-Acetamido-3-hydroxybenzoic acid synthesis
- Product Name:4-Acetamido-3-hydroxybenzoic acid
- CAS Number:10098-40-5
- Molecular formula:C9H9NO4
- Molecular Weight:195.17
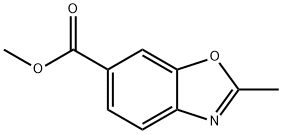
136663-23-5
57 suppliers
$32.00/250mg

10098-40-5
38 suppliers
$85.00/50mg
Yield: 100%
Reaction Conditions:
Stage #1:methyl 2-methyl-1,3-benzoxazole-6-carboxylate with methanol;potassium hydroxide;water in tetrahydrofuran at 20; for 1.08333 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;methanol;water; pH=2.5
Steps:
13a.2
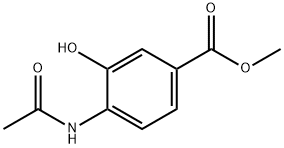
Step 2: 2-Methylbenzord1oxazole-6-carboxyric acid (139); [0742] A solution of compound 138 (920 mg, 4.815 mmol) in THF: MeOH (30 mL of a 1 :1 solution) was treated with a solution of potassium hydroxide (0.698 g, 17.86 mmol) in H2O (15 mL). The reaction mixture was stirred for 65 min at room temperature then quenched with INHCl (18 mL, 18.6 mmol) (final pH of 2.5), THF was removed under reduced pressure and the remaining aqueous solution was cooled to -78 0C and lyophilized to give compound 139 (940 mg, quantitative).
References:
METHYLGENE INC. WO2007/118137, 2007, A1 Location in patent:Page/Page column 109
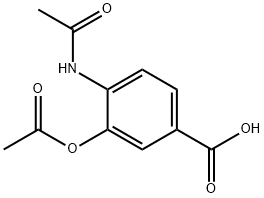
39267-53-3
11 suppliers
$490.00/1g

10098-40-5
38 suppliers
$85.00/50mg
162252-43-9
3 suppliers
inquiry

10098-40-5
38 suppliers
$85.00/50mg

2374-03-0
324 suppliers
$12.72/1gm:

75-36-5
563 suppliers
$17.92/100G

10098-40-5
38 suppliers
$85.00/50mg
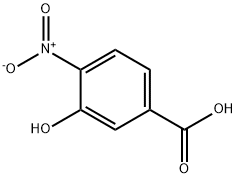
619-14-7
292 suppliers
$10.00/5g

10098-40-5
38 suppliers
$85.00/50mg