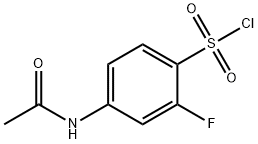
4-(acetylamino)-2-fluorobenzenesulfonyl chloride synthesis
- Product Name:4-(acetylamino)-2-fluorobenzenesulfonyl chloride
- CAS Number:344-70-7
- Molecular formula:C8H7ClFNO3S
- Molecular Weight:251.66
351-28-0
163 suppliers
$10.00/5g

344-70-7
13 suppliers
inquiry
Yield:344-70-7 47%
Reaction Conditions:
with chlorosulfonic acid in water at 0 - 75;
Steps:
1.6 Preparation 6: Synthesis of 4-amino-2-fluoro-N-methylbenzenesulfonamide
[A] solution of N- (3-fluorophenyl) acetamide (20.0, 0.13 mol) in [CHLOROSULFONIC] acid (150 mL) was heated to [75 C] for 1 hr. The solution was allowed to cool to room temperature and was poured over ice. The resulting slurry was extracted with dichloromethane. The combined organic extracts were dried over magnesium sulfate, filtered and evaporated to yield 15.5 g (47%) of 4-acetylamino-2- [FLUOROBENZENESULFORIYL CHLORIDE] as a paste. The crude material was used in the subsequent step without further purification. The above prepared 4-acetylamino-2-fluorobenzenesulfonyl chloride (2 g, 7.9 mmol) was dissolved in [DICHLOROMETHANE] and methylamine (9.9 mL of a 2M solution in THF, 19.9 mmol) was added. The reaction was stirred at room temperature for 2 hrs. The solvent was then evaporated under reduced pressure and the resulting solid was suspended in 50 mL of water. The solid was isolated by filtration and dried to yield 1.55 g (79%) of N- (3-fluoro-4-methylsulfamoylphenyl) acetamide which was used in the subsequent step without further purification. The above prepared [N- (3-FLUORO-4-METHYLSULFAMOYLPHENYL)] acetamide (1.55 g, 6.3 mmol) was suspended in 40 mL of 6M HCI and heated to reflux for 1 hr. The reaction was cooled in an ice bath and an [NAOH] solution was added to adjust the mixture to pH 5. The resulting white precipitate was isolated by filtration and was washed with water and dried to yield 1.07 g (83%) of the title compound. MS [(M/Z,] ES+): 205.0 [(M+1,] 100%) [; H] NMR (500 MHz, ppm, [DMSO-D6)] : 7.35 (m, 1H), 7.12 (m, [1 H),] 6.40 (m, 2H), 6.23 (s, 2H), 2.40 (s, 3H).
References:
WO2004/11460,2004,A2 Location in patent:Page 58
372-19-0
320 suppliers
$10.00/1g

344-70-7
13 suppliers
inquiry