
4-(acetylamino)-3-methylbenzoic acid synthesis
- Product Name:4-(acetylamino)-3-methylbenzoic acid
- CAS Number:37901-92-1
- Molecular formula:C10H11NO3
- Molecular Weight:193.2
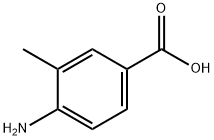
2486-70-6
396 suppliers
$5.00/1g

108-24-7
5 suppliers
$14.00/250ML

37901-92-1
33 suppliers
$31.00/250mg
Yield:37901-92-1 93%
Reaction Conditions:
Stage #1: 4-amino 3-methylbenzoic acidwith triethylamine in dichloromethane; for 0.5 h;
Stage #2: acetic anhydride in dichloromethane at 20; for 72 h;
Steps:
4-Acetamido-3-methylbenzoic acid II
Et3N (25.09 mL, 180 mmol)was added dropwise to 4-amino-3-methylbenzoic acid I (9 g, 60 mmol) dissolved in CH2Cl2 (100 mL) in a 250 mL flask. After 0.5 h, Ac2O was injected slowly. The reaction mixture was vigorously stirred at room temperature for 3 days. The mixture was evaporated to remove the solvent, and the residues were redissolved in 1 M hydrochloric acid (50mL) and water (100 mL). Overnight, light-pink long crystals grew and were filtered off (10.7 mg, 93%); m.p. 237-239 (lit.11 114-116 °C);IR (KBr cm-1): 3270, 1662, 1522, 1422, 1279; 1H NMR (DMSO-d6): δ12.79 (s, 1H), 9.40 (s, 1H), 7.78 (s, 1H), 7.73 (s, 2H), 2.27 (s, 3H), 2.11(s, 3H); ESI: m/z = 386.7 [M+H]+ (double).
References:
Han, Xiao-Feng;Chen, Man-Man;Zhou, Zhi-Mimg [Journal of Chemical Research,2015,vol. 39,# 7,p. 407 - 409]

2486-70-6
396 suppliers
$5.00/1g

37901-92-1
33 suppliers
$31.00/250mg
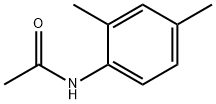
2050-43-3
56 suppliers
$15.00/5mg

37901-92-1
33 suppliers
$31.00/250mg
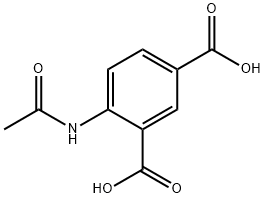
7501-68-0
1 suppliers
inquiry
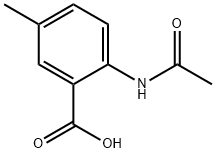
67081-68-9
17 suppliers
inquiry

2486-70-6
396 suppliers
$5.00/1g

108-24-7
5 suppliers
$14.00/250ML

64-19-7
1536 suppliers
$10.00/25ML

37901-92-1
33 suppliers
$31.00/250mg