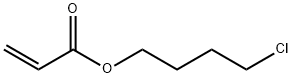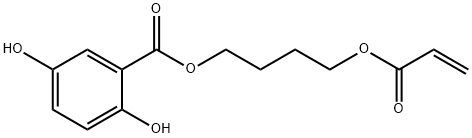
4-(Acryloyloxy)butyl 2,5-Dihydroxybenzoate synthesis
- Product Name:4-(Acryloyloxy)butyl 2,5-Dihydroxybenzoate
- CAS Number:1401243-64-8
- Molecular formula:C14H16O6
- Molecular Weight:280.27
Yield:1401243-64-8 90%
Reaction Conditions:
with potassium hydrogencarbonate in N,N-dimethyl-formamide at 50; for 24 h;
Steps:
Solid KHCO3 (3.0 g, 30 mmol) was added to a stirred mixture of 4-chloro-1-butanolacrylate (3.2 g, 20 mmol) and 2,5-dihydroxybenzoic acid (3.8 g, 25 mmol) in DMF(100 mL). The mixture was heated to 50°C and stirred for 24 h. The reaction mixturewas cooled down to room temperature, diluted with water (100 mL), and extractedtwice with 50 mL DCM. The organic phases were washed twice with water (50 mL) anddried over MgSO4. After evaporation of the solvents, the residue was subjected to columnchromatography on silica gel with DCM as eluting solvent to yield white powder (90%).1H NMR (600 MHz, d6-DMSO) δ (ppm): 9.99 (b, 1H, OH), 9.20 (b, 1H, OH), 7.13 (d, 1H,ArH), 6.98 (m, 1H, ArH), 6.82 (d, 1H, ArH), 6.34, 6.19, 5.94 (3m, 3H, CH2=CH), 4.34, 4.19(2t, 4H, -CH2-O), 1.79 (m, 4H, -CH2-). 13CNMR(150MHz, d6-DMSO) δ (ppm): 25.3, 33.8,64.2, 65.2, 113.0, 114.6, 118.7, 124.4, 128.7, 132.2, 150.1, 153.9, 166.0 and 169.5.
References:
Wei, Renbo;Hua, Xiufu [Molecular Crystals and Liquid Crystals,2017,vol. 643,# 1,p. 83 - 96]
![2-Propenoic acid, 4-[(methylsulfonyl)oxy]butyl ester](/CAS/20210305/GIF/561015-28-9.gif)
561015-28-9
0 suppliers
inquiry

490-79-9
487 suppliers
$5.00/100mg

1401243-64-8
11 suppliers
inquiry

490-79-9
487 suppliers
$5.00/100mg

1401243-64-8
11 suppliers
inquiry
2150-46-1
227 suppliers
$6.00/1g

1401243-64-8
11 suppliers
inquiry