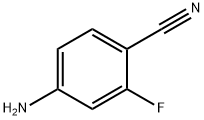
4-AMINO-2-FLUOROBENZONITRILE synthesis
- Product Name:4-AMINO-2-FLUOROBENZONITRILE
- CAS Number:53312-80-4
- Molecular formula:C7H5FN2
- Molecular Weight:136.13
Yield: 72%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in N,N-dimethyl-formamide at 26 - 130; for 16 h;Inert atmosphere;
Steps:
Intermediate 100: 4-amino-2-fluorobenzonitrile:
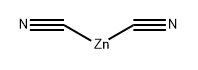
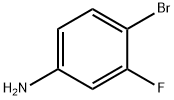
To a solution of 4-bromo-3-fluoroaniline (5.0 g, 26.46 mmol, commercial source: Matrix) in N,N- dimethylformamide (50 mL), Zn(CN)2(9.3 g, 79.38 mmol, commercial source: Sigma Aldric ) was added at 26 °C. The reaction mixture was purged with nitrogen for 10 min, tetrakis(triphenylphosphine)palladium(0) (6.1 g, 5.29 mmol, commercial source: Alfa Aesar) was added and purged again with nitrogen for 10 minutes at 26 °C. The resultant reaction mixture was stirred at 130 °C for 16 h. Upon completion, the reaction mixture was cooled to 26 °C and diluted with ethyl acetate (2x500 mL). The organic layer was washed with water (2x100 mL), dried over anhydrous Na2S04, filtered and the filtrate was evaporated under reduced pressure. The crude was purified by column chromatography using silica (100-200 mesh), eluted with 15% ethyl acetate in petroleum ether. The pure fractions were concentrated under reduced pressure to afford 4- amino-2-fluorobenzonitrile (2.7 g, 72%) as an off white solid.1H NMR (400 MHz, CDCI3) δ 7.38- 7.31 (m, 1 H), 6.47-6.38 (m, 2H), 4.26 (br s, 2H). MS m/z [M+H]+= 137.06
References:
GLAXOSMITHKLINE INTELLECTUAL PROPERTY DEVELOPMENT LIMITED;ALEMPARTE-GALLARDO, Carlos;ENCINAS, Lourdes;ESQUIVIAS PROVENCIO, Jorge WO2019/34729, 2019, A1 Location in patent:Page/Page column 83
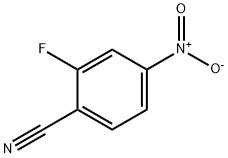
34667-88-4
124 suppliers
$10.00/1g

53312-80-4
136 suppliers
inquiry

1338209-74-7
0 suppliers
inquiry

53312-80-4
136 suppliers
inquiry
1427-07-2
345 suppliers
$6.00/5g

53312-80-4
136 suppliers
inquiry