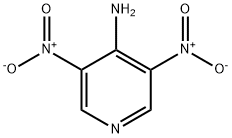
4-AMINO-3,5-DINITROPYRIDINE synthesis
- Product Name:4-AMINO-3,5-DINITROPYRIDINE
- CAS Number:31793-29-0
- Molecular formula:C5H4N4O4
- Molecular Weight:184.11
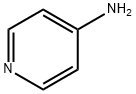
504-24-5
474 suppliers
$10.00/1 g

31793-29-0
83 suppliers
inquiry
Yield: 75%
Reaction Conditions:
Stage #1:4-aminopyridine with sulfuric acid;nitric acid at 0 - 85; for 1.5 h;
Stage #2: with sodium carbonate
Steps:
1
Example 1 3,5-Dinitro-pyridin-4-ylamine (2). H2SO4 (20 ml) was cooled in an ice bath to 0° C. 4-aminopyridine 1 (4.84 g, 51.5 mmol) was added slowly to the acid. Fuming nitric acid (2.7 ml) was added dropwise, taking care to maintain a temperature below 10° C. This mixture was allowed to warm to room temperature over the course of 1 hour. The mixture was then heated to 85° C., and an additional amount of fuming nitric acid (2.7 ml) was added slowly. After 30 minutes, the mixture was allowed to cool to room temperature. The acid mixture was neutralized with Na2CO3 and extracted repeatedly with ethyl acetate. The combined ethyl acetate washings were dried over MgSO4, and concentrated on a rotary evaporator to yield the title product 2, an orange oil (7.1 g (75%), 38.6 mmol). 1H NMR (DMSO-d6): δ 9.21(s,2H), 8.73 (bs, 2H). MS (ESI-POS): [M+H]+=185.
References:
WYETH US2006/189616, 2006, A1 Location in patent:Page/Page column 13; 19

504-24-5
474 suppliers
$10.00/1 g
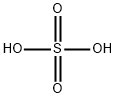
7664-93-9
6 suppliers
$21.64/1003
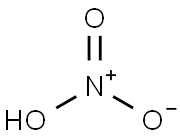
7697-37-2
8 suppliers
$13.00/25g

31793-29-0
83 suppliers
inquiry