
(4-AMINO-3-BROMO-PHENYL)-ACETONITRILE synthesis
- Product Name:(4-AMINO-3-BROMO-PHENYL)-ACETONITRILE
- CAS Number:882855-96-1
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
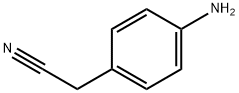
3544-25-0
290 suppliers
$10.00/5g

882855-96-1
30 suppliers
inquiry
Yield:882855-96-1 92%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 0 - 20;
Steps:
52.a
Example 52; 5-Cyano- 1 H-imidazole-2-carboxylic acid [2-(4 j4-dimethyl-cyclohex- 1 -enyl)-4-( 1 H-tetrazol-5 - ylmethyl)-phenyl] -amide; a) (4-Amino-3-bromo-phenyl)-acetonitrile; A solution of 4-aminophenylacetonitrile (1.45 g, 10.9 mmol) in acetonitrile (10 mL) at 00C was treated with NBS (1.95 g, 10.9 mmol) in acetonitrile (10 mL) dropwise via an
References:
WO2007/123516,2007,A1 Location in patent:Page/Page column 113-114
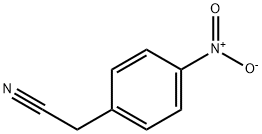
555-21-5
43 suppliers
$21.00/25g

882855-96-1
30 suppliers
inquiry