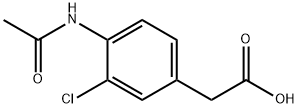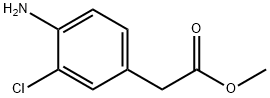
(4-Amino-3-chloro-phenyl)-acetic acid methyl ester synthesis
- Product Name:(4-Amino-3-chloro-phenyl)-acetic acid methyl ester
- CAS Number:101349-30-8
- Molecular formula:C9H10ClNO2
- Molecular Weight:199.63
Yield: 48%
Reaction Conditions:
with acetic acid in methanol;water
Steps:
84 4-[1-[3-chloro-4-[1N'-(2-methylphenyl)ureido]phenylacetyl]-(4S)-fluoro-(2S)-pyrrolidinyl]methoxybenzoic Acid
A mixture of methyl 3chloro-4-nitrophenylacetate (10.9 g, 47.5 mmol), reduced iron powder (8.58 g, 153.6 mmol), AcONa.3H2O (6.05 g, 44.5 mmol) and AcOH (17.6 ml) in MeOH/H2O (100/400 ml) was heated at 110° C. for 1 h. After cooled to room temperature, the reaction mixture was filtered through Celite and the filtered cake was washed with MeOH. The combined filtrate were evaporated and extracted with EtOAc. The combined extracts were washed with brine, dried over Na2SO4 and concentrated in vacuo. The residue was chromatographed on silica gel [150 g, CHCl31EtOAc (10/1)] to give methyl 4-amino-3-chlorophenylacetate (4.58 g, 48%) as a red oil. 1H-NMR (CDCl3) δ 3.49 (s, 2H), 3.68 (s, 3H), 4.01 (br, 2H), 6.70 (d, J=7.4 Hz, 1H), 6.96 (dd, J=8.1, 2.0 Hz, 1H), 7.17 (d, J=2.0 Hz, 1H).
References:
Daiichi Pharmaceutical Co., LTD. US2003/78249, 2003, A1

97522-06-0
2 suppliers
inquiry

101349-30-8
6 suppliers
inquiry
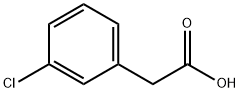
1878-65-5
305 suppliers
$14.00/25g

101349-30-8
6 suppliers
inquiry

53088-68-9
102 suppliers
$18.00/5g

101349-30-8
6 suppliers
inquiry