
(4-AMINO-3-FLUORO-PHENYL)-METHANOL synthesis
- Product Name:(4-AMINO-3-FLUORO-PHENYL)-METHANOL
- CAS Number:146019-45-6
- Molecular formula:C7H8FNO
- Molecular Weight:141.14
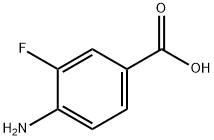
455-87-8
217 suppliers
$6.00/1g

146019-45-6
41 suppliers
$41.00/100mg
Yield:146019-45-6 48.4%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Steps:
1 Step-I: Preparation of (4-Amino-3 -fi uorophenyl)methanol (1 Sb)
To a suspension of lithium aluminum hydride (I .835 g, 48.3 rnmol) in THF (20 mL) was added dropwise at 0°C a solution of 4-arnino-3-fiuorobenzoic acid (5 g, 32.2 mrnol) in THF (20 mL). The reaction mixture was stirred at room temperature overnight. The mixturewas then cooled down to 00 C, quenched with ethyl acetate (30 rnL) and water (10 mL). The slurry obtained was filtered through Celite and washed with ethyl acetate (50 mL). The aqueous layer was separated and organic layer was dried, filtered and concentrated in vacuum to dryness to give crude product. The crude was purified by flash column chromatography (silica gel 80 g, eluting with 0-100% ethyl acetate in hexane) to furnish (4-Arnino-3-fluorophenyl)methanol (15b) (2.2 g, 48.4 % yield) as a tan solid; ‘H NMR (300MHz, DMSO-d6) 6.91 (dd,J 12.5, 1.8 Hz, III), 6.81 (dd,J 8.1, 1.8 Hz, 1H), 6.70(dd,J 9.3, 8.0 Hz, I H), 5.03 - 4.93 (m, 3H), 4.31 (d, J = 5.5 Hz, 2H); MS (ES+) 142.0 (M+ 1);(ES-) 140.0 (M-1).
References:
WO2015/134998,2015,A1 Location in patent:Page/Page column 151; 152
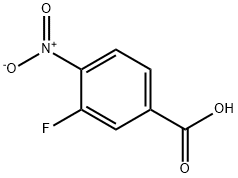
403-21-4
253 suppliers
$6.00/1g

146019-45-6
41 suppliers
$41.00/100mg