
4-AMINO-3-METHYLBIPHENYL, HYDROCHLORIDE synthesis
- Product Name:4-AMINO-3-METHYLBIPHENYL, HYDROCHLORIDE
- CAS Number:3419-49-6
- Molecular formula:C13H14ClN
- Molecular Weight:219.71
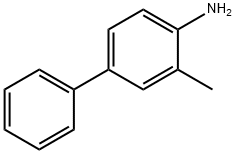
63019-98-7
32 suppliers
$150.00/100mg

3419-49-6
14 suppliers
inquiry
Yield:3419-49-6 37 g
Reaction Conditions:
with hydrogenchloride in n-heptane at 20; for 1 h;Inert atmosphere;Cooling with ice;
Steps:
5
A nitrogen gas atmosphere was prepared in a reaction vessel, then, the compound 5a (92 g) and cyclopentyl methyl ether (214 mL) were added, and the mixture was stirred. Thereafter, the reaction vessel was cooled using an ice bath, and a 16% by mass hydrogen chloride cyclopentyl methyl ether solution (114 g) was dropped, then, n-heptane (649 mL) was dropped. After dropping, the mixture was stirred at room temperature for 1 hour, the deposited solid was filtrated, and the resultant solid was washed with n-heptane and acetone. The resultant solid was recrystallized twice using a mixed solvent of 2-propanol, methanol, ethanol and n-heptane, then, recrystallized once using a mixed solvent of 2-propanol, ethanol and n-heptane, and the resultant solid was dried at room temperature under reduced pressure, to obtain a compound 5b (37 g, pale red solid). The above-described operation was repeated, thereby ensuring a necessary amount of the compound 5b. The measurement results of NMR of the compound 5b were as described below. 1H-NMR (400 MHz, CDCl3) δ (ppm)=7.33-7.65 (8H, m), 4.85 (3H, s), 2.46 (3H, s).
References:
US2017/283383,2017,A1 Location in patent:Paragraph 0229; 0231-0233
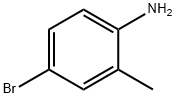
583-75-5
300 suppliers
$5.00/1g

3419-49-6
14 suppliers
inquiry

24388-23-6
209 suppliers
$6.00/5g

3419-49-6
14 suppliers
inquiry