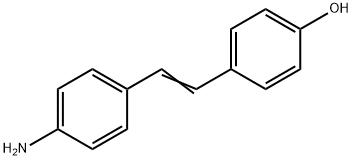
4'-AMINO-4-HYDROXYSTILBENE synthesis
- Product Name:4'-AMINO-4-HYDROXYSTILBENE
- CAS Number:836-44-2
- Molecular formula:C14H13NO
- Molecular Weight:211.26
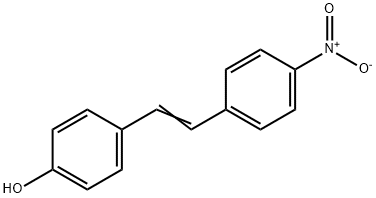
19221-08-0
30 suppliers
$53.00/1g

836-44-2
18 suppliers
inquiry
Yield:836-44-2 76.1%
Reaction Conditions:
with sodiumsulfide nonahydrate in water; for 6 h;Reflux;
Steps:
4-Nitro-4’-hydroxyazobenzene (17.98 g, 73.99mmol) was dissolved under reflux in 650 ml of ethanoland a hot solution containing 53.3 g (222.08 mmol) sodiumsulfide nonahydrated salt, 100 ml of water and 100ml of ethanol was added over. The mixture was heated underreflux for 6 hours. After cooling, the mixture was progressivelyneutralized using concentrated hydrochloric acid to pH = 7. The yellow solid was collected by filtrationand washed several times with water. 4-Amino-4’-hydroxyazobenzene was further used without prior purification.Yellow crystals, Yield: 12 g (76.1%). 1H-NMR(DMSO, 400 MHz): δ 8.83 (s, 1H, OH), 7.76 (d, 2H,ArH), 7.71 (d, 2H, ArH), 6.98 (d, 2H, ArH), 6.79 (d, 2H,ArH), 5.30 (s, 2H, NH2). 13C-NMR (DMSO, 100 MHz): δ160.27, 152.55, 147.70, 145.38, 125.60, 124.98, 116.72,114.96 (8C, aromatic).
References:
Onofrei, Roxana Maria;Carlescu, Irina;Epure, Luiza;Scutaru, Dan [Acta Chimica Slovenica,2013,vol. 60,# 3,p. 604 - 616]
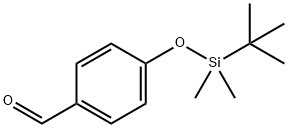
120743-99-9
36 suppliers
$59.50/1g

836-44-2
18 suppliers
inquiry

123-08-0
906 suppliers
$5.00/10g

836-44-2
18 suppliers
inquiry