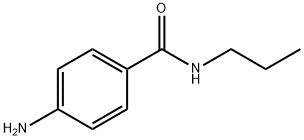
4-AMINO-N-PROPYLBENZAMIDE synthesis
- Product Name:4-AMINO-N-PROPYLBENZAMIDE
- CAS Number:38681-78-6
- Molecular formula:C10H14N2O
- Molecular Weight:178.23
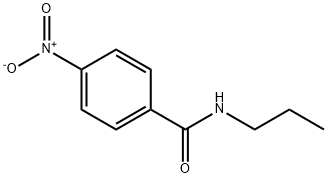
2585-24-2
25 suppliers
$109.00/25g

38681-78-6
15 suppliers
$85.00/250mg
Yield:38681-78-6 91%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in ethanol;ethyl acetate at 50;
Steps:
3
Preparation No.3. 4-Amino-N-propylbenzamide4-Nitro-A -propylbenzamide (3.31 g, 15.9 mmol) was dissolved in EtOAc (125 mL) and EtOH (125 mL) and passed through the H-Cube at 1 mL/min equipped with a 10% Pd/C catcart (ThalesNano), at full hydrogen and temperature set to about 50 °C. The solvent was stripped off and the solid was dried overnight in a vacuum oven set at about 50 °C to provide 4-amino- V- propylbenzamide (2.58 g, 91%): .H NMR (DMSO-d?) δ 7.93 (t, J = 5.48 Hz, 1H); 7.54 (d, J = 8.57 Hz, 2H); 6.51 (d, J = 8.67 Hz, 2H) 5.54 (br s, 2H) 3.13 (m, 2H) 1.47 (sextet, J2H) 0.85 (t, J = 8.49 Hz, 3H).
References:
WO2012/48222,2012,A1 Location in patent:Page/Page column 99-100
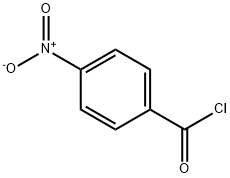
122-04-3
8 suppliers
$15.00/1g

38681-78-6
15 suppliers
$85.00/250mg