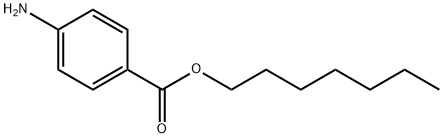
4-Aminobenzoic acid heptyl ester synthesis
- Product Name:4-Aminobenzoic acid heptyl ester
- CAS Number:14309-40-1
- Molecular formula:C14H21NO2
- Molecular Weight:235.32
Yield:14309-40-1 43 %
Reaction Conditions:
with potassium carbonate in acetone at 20;
Steps:
3.2. Material and methods
General procedure: In a round bottom flask, starting substrate, appropriate alkyl halides and K2CO3 wereadded (1:2:1) in the presence of acetone. The reaction mixture was stirred for 5-6 h and the progress of the reaction was examined with the help of thin layer chromatogragphy(TLC). After completion of reaction, the reaction mixture was treated with dichloromethane and water (1:1) and put into separatory funnel to separate organicand aqueous layer. Then the organic layer was dried over Na2SO4 and freed of thesolvent under vacuum. The mono and dialkylated products were purified using column chromatography (CC) in a solvent system of petroleum ether and ethyl acetate(7:3 and 8:2, respectively). The monoalkylated derivatives (2-11) are obtained in higheryield than dialkylated derivatives (12-21), about 50 and 30% respectively.
References:
Hasan, Erum;Ali, Syed Nawazish;Bano, Zarina;Begum, Sabira;Ali, Sundus;Shams, Afshan;Siddiqui, Bina S. [Natural Product Research,2023]
14309-44-5
0 suppliers
inquiry

14309-40-1
9 suppliers
inquiry