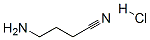
4-aminobutyronitrile monohydrochloride synthesis
- Product Name:4-aminobutyronitrile monohydrochloride
- CAS Number:16011-90-8
- Molecular formula:C4H9ClN2
- Molecular Weight:120.5807

32754-99-7
13 suppliers
inquiry
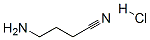
16011-90-8
18 suppliers
inquiry
Yield: 85%
Reaction Conditions:
with hydrogenchloride in chloroform at 4; for 0.333333 h;Inert atmosphere;
Steps:
Synthesis of 4-aminobutanenitrile hydrochloride (1.HCl)
1 (116 mg, 1.38 mmol, 1 equiv) was dissolved in chloroform (2 mL) in a two neck round bottom flask equipped with a drying tube. Hydrochloric acid gas was produced through slow addition of H 2 SO 4 onto NH 4 Cl and bubbled through the reaction solution, carried by a stream of N 2 gas, for 20 mins while cooling at 4°C. Solvent was removed under an N 2 stream and the residue dried under reduced pressure for 2 h to yield 1.HCl as a white solid (142 mg, 85%). 1 H NMR in D 2 O was in agreement with literature 15 : 1 H NMR (599 MHz, D 2 O) δ 3.13 (t, J = 7.6 Hz, 2H), 2.65 (t, J = 7.2 Hz, 2H), 2.11 - 2.02 (m, 2H) ppm. 1 H NMR (500 MHz, DMSO-d 6 ) δ 8.13 (s, 3H), 2.89 - 2.77 (m, 2H), 2.65 (t, J = 7.2 Hz, 2H), 1.88 (p, J = 7.3 Hz, 2H) ppm. 13 C NMR (126 MHz, DMSO-d 6 ) δ 123.02, 40.72, 26.15, 16.97 ppm. FTIR (ATR mode): 3450 (N-H), 3013 (N-H), 2841 (C-H), 2250 (CN), 1656 (N-H) cm -1 .
References:
Capon, Patrick K.;Avery, Thomas D.;Purdey, Malcolm S.;Abell, Andrew D. [Synthetic Communications,2021,vol. 51,# 3,p. 428 - 436] Location in patent:supporting information
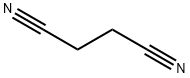
110-61-2
230 suppliers
$13.00/5g
25150-61-2
29 suppliers
$25.00/250mg
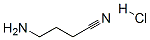
16011-90-8
18 suppliers
inquiry
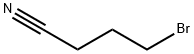
5332-06-9
145 suppliers
$10.00/1g
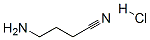
16011-90-8
18 suppliers
inquiry
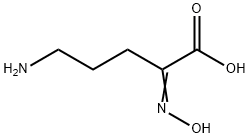
146952-04-7
0 suppliers
inquiry
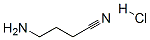
16011-90-8
18 suppliers
inquiry