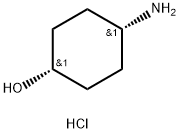
4-aminocyclohexan-1-ol synthesis
- Product Name:4-aminocyclohexan-1-ol
- CAS Number:56239-26-0
- Molecular formula:C6H14ClNO
- Molecular Weight:151.63
![2-Oxa-3-aza-bicyclo[2.2.2]oct-5-ene hydrochlorate](/CAS/GIF/56239-25-9.gif)
56239-25-9
14 suppliers
inquiry

56239-26-0
181 suppliers
$6.00/250mg
Yield:-
Reaction Conditions:
Stage #1:(+/-)-2-Oxa-3-azabicyclo[2.2.2]oct-5-ene hydrochloride with hydrogen;platinum(IV) oxide under 1500.15 Torr; for 7 h;
Stage #2: with hydrogenchloride in 1,4-dioxane
Steps:
a
To a solution of 30.0 g (0.265 mol) of cyclohexanone oxime in 300 ml of methylene chloride and 38 ml of ethanol was slowly added at 0 0C 34.5 g (0.318 mol) of tert- butyl-hypochlorite. The resulting dark blue solution was cooled to -2O0C and then 31.9 g (0.398 mol) of 1 ,3-cyclohexadiene were added and the mixture was stored in a freezer at 5°C for 2 days until the blue color had disappeared. The reaction mixture was concentrated to 50% of its volume and then 600 ml of diethyl ether were slowly added. After stirring overnight the resulting precipitate was isolated by suction to yield 29,0 g of 2-oxa-3-aza-bicyclo[2.2.2]oct-5-ene hydrochloride. 5.0 g (0.045 mol) of this material were hydrogenated with 3.0 g (0.013 mol) platinum oxide at 2 bar hydrogen pressure. After 7 h the catalyst was filtered off and a solution of 20 ml 4 M hydrochloric acid in dioxane was added. After evaporation the residue was recrystallized from 30 ml isopropanol giving 3.1 g of cis-4-aminocyclohexanol hydrochloride.
References:
SANOFI-AVENTIS DEUTSCHLAND GMBH WO2007/12422, 2007, A1 Location in patent:Page/Page column 82-83