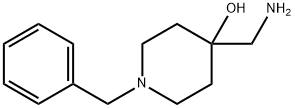
4-(aminomethyl)-1-benzylpiperidin-4-ol synthesis
- Product Name:4-(aminomethyl)-1-benzylpiperidin-4-ol
- CAS Number:23804-68-4
- Molecular formula:C13H20N2O
- Molecular Weight:220.31
![6-benzyl-1-oxa-6-azaspiro[2,5]octane](/CAS/GIF/19867-34-6.gif)
19867-34-6
42 suppliers
inquiry

23804-68-4
29 suppliers
$90.00/50mg
Yield:23804-68-4 89%
Reaction Conditions:
with ammonium hydroxide in methanol;water monomer at 20;
Steps:
26 Preparation 26: 4-(aminomethyl)-l-benzylpiperidin-4-ol (P26)
To a stirred solution of 6-benzyl-l-oxa-6-azaspiro[2.5]octane (P8 ,3 g, 14.7 mmol) in MeOH (18 mL), at 0 °C, 28% aq. N H4OH (36 m L) was added. Once the addition was complete, the ice-bath was removed and the resulting reaction mixture was stirred at T overnight. Then, reaction mixture was concentrated under reduced pressure, the residue was taken up with DCM and IN NaOH, aqueous phase was back extracted with DCM, combined organics were dried and concentrated under reduced pressure. 4- (aminomethyl)-l-benzylpiperidin-4-ol (P26, 2.9 g, y= 89%) was obtained as colourless oil. Used as such in next step MS (ES) (m/z): 221.10 [M+H]+.
References:
WO2016/42453,2016,A1 Location in patent:Page/Page column 72