
4-(aminomethyl)-2,6-dimethylphenol hydrochloride synthesis
- Product Name:4-(aminomethyl)-2,6-dimethylphenol hydrochloride
- CAS Number:937-36-0
- Molecular formula:C9H14ClNO
- Molecular Weight:187.67

4198-90-7
245 suppliers
$6.00/5g

937-36-0
5 suppliers
inquiry
Yield:937-36-0 100%
Reaction Conditions:
Stage #1: 3,5-dimethyl-4-hydroxybenzonitrilewith borane-THF in tetrahydrofuran; for 16 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; for 0.5 h;Heating / reflux;
Steps:
54
A solution of borane in tetrahydrofuran (1M in tetrahydrofuran, 27.1 mL, 27.1 mmol) was added dropwise to a solution of 3, 5-dimetNyl-4-hydroxybenzonitrile (1.0 g, 6.79 mmol) in tetrahydrofuran (70 mL) and the resulting solution was heated under reflux for 16 hours. The reaction mixture was cooled to room temperature, treated with 6N hydrochloric acid (20mL) and heated under reflux for a further 30 minutes. The reaction mixture was then cooled to room temperature and the solvent was removed in vacuo. rhe residue was purified using a strong cation exchange resin eluting with methanol followed by 2M ammonia in methanol, to give an orange oil. This oil was then treated with 1M hydrogen chloride in-methanol (20mL) and the reaction mixture was concentrated in vacuo to afford the title compound as a pale yellow solid in quantitative yield, 1.12 g. HNMR (400MHz, CDCI3) 8 : 2.22 (6H, s), 3.75 (2H, s), 6. 90 (2H, s).
References:
WO2005/92840,2005,A1 Location in patent:Page/Page column 75
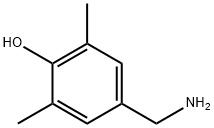
876-15-3
65 suppliers
inquiry

937-36-0
5 suppliers
inquiry