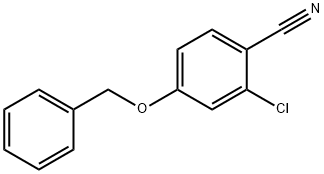
4-BENZYLOXY-3-CHLORO-BENZONITRILE synthesis
- Product Name:4-BENZYLOXY-3-CHLORO-BENZONITRILE
- CAS Number:853953-30-7
- Molecular formula:C14H10ClNO
- Molecular Weight:243.69
3336-16-1
110 suppliers
$13.00/250mg

100-39-0
434 suppliers
$10.00/10g

853953-30-7
9 suppliers
inquiry
Yield:853953-30-7 99%
Reaction Conditions:
with potassium carbonate in acetonitrile at 20; for 18 h;
Steps:
50 4-(Benzyloxy)-2-chlorobenzonitrile
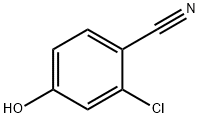
Potassium carbonate (66.3 g, 480 mmol) was added to a mixture of 2-chloro-4-hydroxybenzonitrile (25 g, 160 mmol) and benzyl bromide (19.3 mL, 161 mmol) in acetonitrile (300 mL) and the mixture was stirred for 18 hours at room temperature. The reaction mixture was then filtered and the filtrate was concentrated in vacuo. Trituration of the residue with heptanes afforded the title compound as an off-white solid in 99% yield, 38.65 g.
References:
US2006/35922,2006,A1 Location in patent:Page/Page column 33

3336-16-1
110 suppliers
$13.00/250mg

100-51-6
1404 suppliers
$5.00/100g

853953-30-7
9 suppliers
inquiry