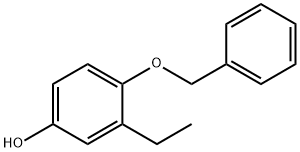
4-Benzyloxy-3-ethyl-phenol synthesis
- Product Name:4-Benzyloxy-3-ethyl-phenol
- CAS Number:188112-41-6
- Molecular formula:C15H16O2
- Molecular Weight:228.29
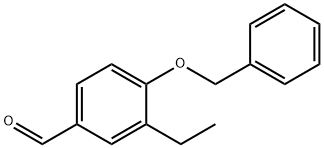
886230-34-8
3 suppliers
inquiry

188112-41-6
4 suppliers
inquiry
Yield:188112-41-6 39%
Reaction Conditions:
Stage #1: 4-benzyloxy-3-ethylbenzaldehydewith ammonium peroxydisulfate;formic acid;phosphoric acid;acetic anhydride in water;toluene at 60; for 20 h;
Stage #2: with toluene-4-sulfonic acid in water;toluene at 20; for 9 h;
Stage #3: with sodium hydrogensulfite in water;toluene; for 0.5 h;
Steps:
4.4
References:
EP1813594,2007,A1 Location in patent:Page/Page column 30-31