
[4-(benzyloxy)-3-fluorophenyl]methanol synthesis
- Product Name:[4-(benzyloxy)-3-fluorophenyl]methanol
- CAS Number:536974-94-4
- Molecular formula:C14H13FO2
- Molecular Weight:232.25
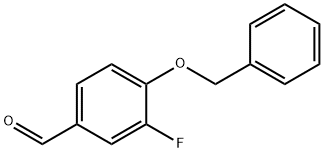
175968-61-3
43 suppliers
$33.00/1g
![[4-(benzyloxy)-3-fluorophenyl]methanol](/CAS/GIF/536974-94-4.gif)
536974-94-4
32 suppliers
$134.00/1g
Yield: 100%
Reaction Conditions:
Stage #1:3-fluoro-4-[(phenylmethyl)oxy]benzaldehyde with sodium tetrahydroborate in tetrahydrofuran at 20;
Stage #2: with hydrogenchloride in tetrahydrofuran;water
Steps:
IV
To a suspension of sodium borohydride (3.4 g, 90 mmol) in THF (60 ml.) was added a solution of 4-benzyloxy-3-fluoro-benzaldehyde (16.1 g, 70 mmol) in THF (60 ml_). After stirring at ambient temperature over night, the reaction mixture was quenched by the addition of ice water. The mixture was acidified with aqueous HCI (4 M) and extracted with Et2O. The combined organic phases were washed with aqueous NaHCO3 solution and dried over sodium sulfate. After removal of the solvent, the product was yielded. Yield: 16.2 g (100%) ESI-MS: m/z = 215 [M-OH]+
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;AJINOMOTO CO., INC.;BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG WO2007/14895, 2007, A2 Location in patent:Page/Page column 33

1379604-20-2
0 suppliers
inquiry
![[4-(benzyloxy)-3-fluorophenyl]methanol](/CAS/GIF/536974-94-4.gif)
536974-94-4
32 suppliers
$134.00/1g
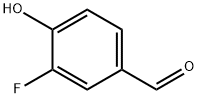
405-05-0
238 suppliers
$12.00/1g
![[4-(benzyloxy)-3-fluorophenyl]methanol](/CAS/GIF/536974-94-4.gif)
536974-94-4
32 suppliers
$134.00/1g

100-39-0
425 suppliers
$10.00/10 g
![[4-(benzyloxy)-3-fluorophenyl]methanol](/CAS/GIF/536974-94-4.gif)
536974-94-4
32 suppliers
$134.00/1g
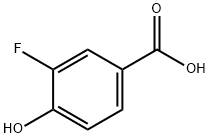
350-29-8
199 suppliers
$13.43/1gm:
![[4-(benzyloxy)-3-fluorophenyl]methanol](/CAS/GIF/536974-94-4.gif)
536974-94-4
32 suppliers
$134.00/1g