
(4-Boc-Amino-piperidin-1-yl)acetic acid ethylester synthesis
- Product Name:(4-Boc-Amino-piperidin-1-yl)acetic acid ethylester
- CAS Number:203662-91-3
- Molecular formula:C14H26N2O4
- Molecular Weight:286.37

105-36-2
342 suppliers
$5.00/5G
73874-95-0
449 suppliers
$10.00/250mg

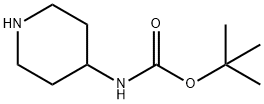
203662-91-3
16 suppliers
$45.00/100mg
Yield:203662-91-3 490 mg
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20;
Steps:
28.1 Step 1: Preparation of ethyl 2-(4-((tert-butoxycarbonyl)amino)piperidin-l-yl)acetate
To a mixture of tert-butyl piperidin-4-yl carbamate (500 mg, 2.49 mmol) in DMF (20 mL) were added ethyl 2-bromoacetate (500 mg, 2.99 mmol) and K2C03(687 mg, 4.98 mmol). The mixture was stirred at r.t. overnight. Water (50 mL) was added, and the mixture was extracted with ethyl acetate (50 mL). The organic extract was washed with brine, dried over anhydrous Na2S04, and evaporated under vacuum. The residue was purified by flash chromatography (silica gel, 1 % ~ 20 % ethyl acetate in petroleum ether) to give ethyl 2-(4- ((tert-butoxycarbonyl)amino)piperidin-l-yl)acetate (490 mg) as a white solid. LC-MS (ESI) found: 287 [M+l]+.
References:
WO2019/118612,2019,A1 Location in patent:Paragraph 0221