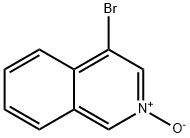
4-Bromo-1-ethoxyisoquinoline synthesis
- Product Name:4-Bromo-1-ethoxyisoquinoline
- CAS Number:59050-59-8
- Molecular formula:C11H10BrNO
- Molecular Weight:252.11
Yield:59050-59-8 98%
Reaction Conditions:
in 1,4-dioxane; for 1 h;Microwave irradiation;Sealed tube;Heating;
Steps:
General procedure for nucleophlic substitution of 1-chloro 4 or 7-bromoisoquinoline with microwave heating.
General procedure: Morpholine (10 mmol), 7-bromo-1-chloroisoquinoline (2 mmol) and 1,4-dioxane (5ml) were dissolved in 10 ml of vial tube. The vial was sealed with a crimp cap and placed in a Biotage initiator microwave cavity. The reaction mixtures were heated 1h at 120 oC. The resulting mixture was evaporated in vacuum. The product was extracted with ethyl acetate. The ethyl acetate layer was dried over anhydrous magnesium sulfate, filtered, and concentrated. The product was purified by silica gel column chromatography using hexane : ethyl acetate = 1:1.7-Bromo-1-(4-morpholinyl)isoqinoline (2a) was obtained in 99 % yield as a brown solid; mp: 129-130; 1H NMR(CDCl3, 300 MHz) δ 8.21 (s, 1H), 8.17 (d, J = 5.7 Hz, 1H), 7.81 (s, 2H), 7.47 - 7.35 (m, 2H), 7.29 (d, J = 5.6 Hz, 1H), 7.10 (t, J = 8.7 Hz, 1H), 4.05 - 3.91 (m, 7H), 3.45 (t, 4H); 13C NMR (75 MHz, CDCl3) δ 160.3, 141.2, 136.6, 133.2, 129.0, 127.8, 122.8, 119.9, 115.9, 67.0, 51.9; MS(m/z) : 293.17 (M+1). The following compounds (2b-f) were prepared with general procedure for nucleophlic substitution of 1-chloro-4 or 7-bromoisoquinoline.
References:
Park, A. Reum;Yum, Eul Kgun [Bulletin of the Korean Chemical Society,2018,vol. 39,# 11,p. 1259 - 1265] Location in patent:supporting information

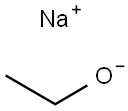
1532-97-4
354 suppliers
$14.00/10g

59050-59-8
4 suppliers
inquiry
3749-21-1
42 suppliers
inquiry

59050-59-8
4 suppliers
inquiry