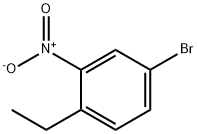
4-bromo-1-ethyl-2-nitrobenzene synthesis
- Product Name:4-bromo-1-ethyl-2-nitrobenzene
- CAS Number:10342-66-2
- Molecular formula:C8H8BrNO2
- Molecular Weight:230.06
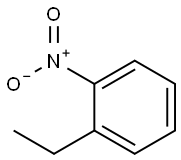
612-22-6
133 suppliers
$14.00/5g

10342-66-2
26 suppliers
inquiry
Yield:10342-66-2 90%
Reaction Conditions:
with N-Bromosuccinimide in dichloromethane; for 2 h;Reflux;
Steps:
1 Synthesis of 2-ethyl-5-bromonitrobenzene
Add 1.1L of dichloromethane and 212g of o-ethylnitrobenzene (1.4mol) to a 3L reaction flask to heat up to reflux, and add 250g of N-bromosuccinimide (1.4mol) in batches under reflux for reflux reaction 2 hours. After the reaction is completed, the temperature is controlled at 25±5°C and 1.27kg of saturated sodium sulfite is added dropwise to the reaction system to terminate the reaction. Adding 0.72 kg of n-heptane to recrystallize to obtain 290.3 g of 2-ethyl-5-bromonitrobenzene with a molar yield of 90%.
References:
CN112538073,2021,A Location in patent:Paragraph 0157-0159
51529-96-5
33 suppliers
inquiry

10342-66-2
26 suppliers
inquiry
1585-07-5
317 suppliers
$6.00/5g

10342-66-2
26 suppliers
inquiry
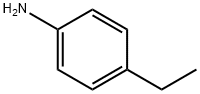
589-16-2
223 suppliers
$10.00/1g

10342-66-2
26 suppliers
inquiry
