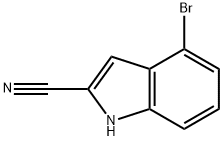
4-Bromo-1H-indole-2-carbonitrile synthesis
- Product Name:4-Bromo-1H-indole-2-carbonitrile
- CAS Number:955978-74-2
- Molecular formula:C9H5BrN2
- Molecular Weight:221.05
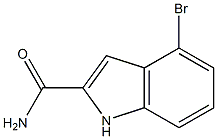
955978-73-1
7 suppliers
inquiry

955978-74-2
40 suppliers
inquiry
Yield: 82%
Reaction Conditions:
with trichlorophosphate in toluene for 0.75 h;Heating / reflux;
Steps:
13
Phosphorous oxychloride (1.9 mL, 20 mmol) was added to a suspension of 4-bromo- lH-indole-2-carboxylic acid amide (1.32 g, 5.5 mmol.) in toluene (10 mL) and the mixture was stirred at reflux for 45 min. On cooling, the mixture was poured into an aqueous Na2CO3 solution (sat., 50 mL) and the mixture stirred until effervescence had subsided. The layers were separated, the aqueous phase extracted with EtOAc and the combined organic layers dried (MgSO4) and evaporated to dryness. The crude material was purified by column chromatography to afford the title compound as a solid (1.00 g, 82 %). NMR δη (400 MHz, CDCl3) 7.22-7.28 (m, 2H), 7.35-7.40 (m, 2H) and 8.79 (s, IH).
References:
Location in patent:Page/Page column 55
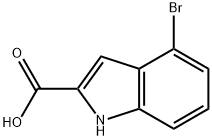
16732-64-2
140 suppliers
$34.00/250mg

955978-74-2
40 suppliers
inquiry