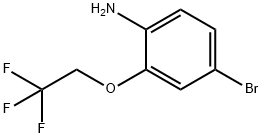
4-Bromo-2-(2,2,2-trifluoroethoxy)-phenylamine synthesis
- Product Name:4-Bromo-2-(2,2,2-trifluoroethoxy)-phenylamine
- CAS Number:1496808-60-6
- Molecular formula:C8H7BrF3NO
- Molecular Weight:270.05
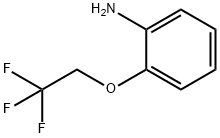
57946-60-8
53 suppliers
$65.00/500mg

1496808-60-6
12 suppliers
inquiry
Yield: 60%
Reaction Conditions:
with bromine in acetic acid at 20; for 1 h;
Steps:
243.3
[00503] Step 3: To a solution of 243b (1.9 g, 10 mmol) in 20 mL of HOAc was added Br2 (2.4 g, 15 mmol, 1.5 eq). The mixture was stirred at rt for 1 h and concentrated. The residue was dissolved in 100 mL of EtOAc and washed with aq. NaHC03 (100 mL X 3). The organic was dried and concentrated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc = 4: 1) to give 243c (1.6 g, 60%) as light yellow solid. ESI- MS (M+H)+: 270.2. 1H NMR (400 MHz, CDC13) δ: 6.99 (dd, J = 1.6 Hz, 8.4 Hz, 1H), 6.89 (d, J = 1.6 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H), 4.34 (q, J = 8.0 Hz, 2H).
References:
BIOGEN IDEC MA INC.;CHAO, Jianhua;ENYEDY, Istvan, J.;GUERTIN, Kevin;HUTCHINGS, Richard, H.;JONES, John, Howard;POWELL, Noel;VANVLOTEN, Kurt, D. WO2014/8214, 2014, A1 Location in patent:Paragraph 00500; 00503
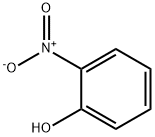
88-75-5
14 suppliers
$15.00/5mg

1496808-60-6
12 suppliers
inquiry
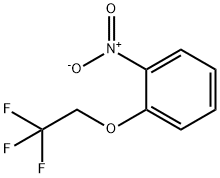
87014-28-6
23 suppliers
$63.00/1 g

1496808-60-6
12 suppliers
inquiry
27684-84-0
153 suppliers
$9.00/1g

1496808-60-6
12 suppliers
inquiry