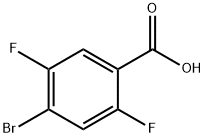
4-bromo-2,5-difluorobenzoic acid synthesis
- Product Name:4-bromo-2,5-difluorobenzoic acid
- CAS Number:28314-82-1
- Molecular formula:C7H3BrF2O2
- Molecular Weight:237
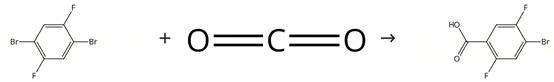 Figure Synthesis of 4-Bromo-2,5-difluorobenzoic acid
Figure Synthesis of 4-Bromo-2,5-difluorobenzoic acid

124-38-9
122 suppliers
$175.00/23402

327-51-5
277 suppliers
$5.00/5g

28314-82-1
142 suppliers
$6.00/250mg
Yield:28314-82-1 97%
Reaction Conditions:
Stage #1:1,4-dibromo-2,5-difluorobenzene with n-butyllithium in diethyl ether;hexane at -78; for 2 h;Inert atmosphere;
Stage #2:carbon dioxide in diethyl ether;hexane at 20; for 1 h;
Steps:
58.a (a) 4-bromo-2,5-difluorobenzoic acid
(a) 4-bromo-2,5-difluorobenzoic acid To a 780C solution of 1,4-dibromo-2,5-difluorobenzene (2.72 g, 9.99 mmol) in dry Et20 (30 mL) under an inert atmosphere was added 2.5 M n-butyllithium solution in hexanes (4 mL, 9.99 mmol) drop-wise and the mixture left stirring for 2 h. Crushed CO2 pellets were added slowly and the mixture was allowed to warm to ambient temperature and left stirring for 1 h. After quenching with 1M aqueous HCI (10 mL) the mixture was basified with 1M aqueous NaOH (70 mL) and then washed with Et20 (2 x 50 mL). The aqueous layer was acidified with 1M aqueous HCI (80 mL) and extracted with Et20 (3 x 100 mL). The organic layer was washed with brine (50 mL), dried over Na2SO4, filtered and solvent was removed in vacuo to give 4-bromo-2,5-difluoro benzoic acid (2.3 g, 97 %) as an off-white solid, which was used without further purification.1H NMR (Method B) (CDCI3): O ppm 9.50 (brs, 1H), 7.78 (dd, J= 8.2, 6.1 Hz, 1H), 7.46 (dd, J= 9.3, 5.4 Hz, 1H); LC-MS (Method C) 234.9/236.9 [M-H] RT 3.43 mm
References:
REDX PHARMA PLC;RATCLIFFE, Andrew;HUXLEY, Anthony;LYTH, David;NOONAN, Gary;KIRK, Ralph;UOSIS-MARTIN, Mario;STOKES, Neil WO2015/155549, 2015, A1 Location in patent:Page/Page column 105; 106
1621165-11-4
2 suppliers
inquiry

28314-82-1
142 suppliers
$6.00/250mg

124-38-9
122 suppliers
$175.00/23402

28314-82-1
142 suppliers
$6.00/250mg

327-51-5
277 suppliers
$5.00/5g

28314-82-1
142 suppliers
$6.00/250mg