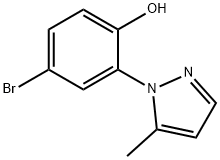
4-bromo-2-(5-methyl-1H-pyrazol-1-yl)phenol synthesis
- Product Name:4-bromo-2-(5-methyl-1H-pyrazol-1-yl)phenol
- CAS Number:1395896-55-5
- Molecular formula:C10H9BrN2O
- Molecular Weight:253.1
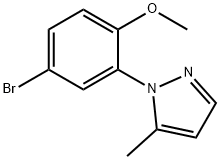
1395896-56-6
5 suppliers
inquiry

1395896-55-5
5 suppliers
inquiry
Yield:1395896-55-5 65%
Reaction Conditions:
Stage #1: 1-(5-bromo-2-methoxyphenyl)-5-methyl-1H-pyrazolewith phenylborondichloride in toluene at 20 - 95; for 3 h;
Stage #2: with sodium hydroxide in water;toluene at 20; for 0.5 h;
Stage #3: with hydrogenchloride in water at 20; pH=9 - 10;
Steps:
2.2
Step 2. Synthesis of 4-bromo-2-(5-methyl-1 /-/-pyrazol-1 -yl)phenol (C15).Dichlorophenylborane (0.51 kg, 3.2 mol) was added at a slow controlled rate to a solution of 1 -(5-bromo-2-methoxyphenyl)-5-methyl-1 H-pyrazole (C14) (0.57 kg, 2.13 mol) in toluene (5.73 L), while maintaining the temperature at 20 °C +/- 10 °C. The reaction mixture was heated to 95 °C +/- 5 °C and held at this temperature for 3 hours, at which time the reaction was deemed complete by HPLC analysis. Aqueous sodium hydroxide solution (1 N, 9.4 L, 9.4 mol) was added at a slow controlled rate while maintaining the temperature at 20 °C +/- 10 °C, and the reaction mixture was stirred for 30 minutes. The organic layer was extracted with 1 N aqueous sodium hydroxide (2.6 L, 2.6 mol). Concentrated aqueous hydrogen chloride (37%) was added to the combined aqueous layers at a slow controlled rate, while maintaining the temperature at 20 °C +/- 5 °C, until a pH of 9-10 was achieved. The resulting solid was collected by filtration, washed with water (2.86 L) and dried under a stream of nitrogen. The wet cake was mixed with isopropyl alcohol (4.58 L) and the slurry was heated to 50 °C +/- 5 °C for 1 hour, at which point all solids had dissolved. The solution was cooled to 20 °C +/- 5 °C and then water (4.58 L) was added slowly over 1 hour. This slurry was held at 20 °C +/- 5 °C for 2 hours, then filtered, washed with water (2.86 L) and dried in a tray dryer under vacuum to afford the title product. The minor pyrazole regioisomer was no longer present, by 1 H NMR analysis. Yield: 352 g, 1 .39 mol, 65% LCMS m/z 253.0, 255.0(M+1 ). 1H NMR (400 MHz, DMSO-cf6) δ 10.40 (s, 1 H), 7.50 (apparent br dd, J=1.8, 0.5 H; likely a broadened dq, J=1 .8, 0.5 Hz; 1 H), 7.47 (dd, J=8.8, 2.5 Hz, 1 H), 7.37 (d, J=2.5 Hz, 1 H), 7.00 (d, J=8.8 Hz, 1 H), 6.17-6.19 (m, 1 H), 2.12-2.13 (m, 3H).
References:
WO2012/172449,2012,A1 Location in patent:Page/Page column 32-33
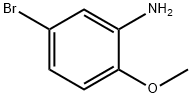
6358-77-6
154 suppliers
$6.00/250mg

1395896-55-5
5 suppliers
inquiry