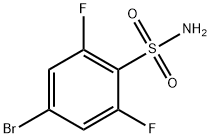
4-Bromo-2,6-difluorobenzene sulfonamide synthesis
- Product Name:4-Bromo-2,6-difluorobenzene sulfonamide
- CAS Number:263349-74-2
- Molecular formula:C6H4BrF2NO2S
- Molecular Weight:272.07
67567-26-4
339 suppliers
$5.00/1g

263349-74-2
8 suppliers
inquiry
Yield:263349-74-2 82%
Reaction Conditions:
Stage #1: 4-bromo-2,6-difluoroanilinewith hydrogenchloride;thionyl chloride in water at 0 - 50; for 1 h;Inert atmosphere;
Stage #2: with sodium nitrite in water at -5 - 0; for 0.166667 h;Inert atmosphere;Further stages;
Steps:
47.1 4-(aminomethyl)-2,6-difluorobenzenesulfonamide HCl
Step 1. To a flask containing water (45 mL, 2.3 M) at 0 C, thionyl chloride (7.5 mL, 103.2 mmol, 4.3 eq) was added dropwise. The resulting mixture was stirred at 22 C for 18 hr.4- Bromo-2,6-difluoroaniline (5 g, 24 mmol, 1 eq) at 0 C was added conc HCl (24 mL, 1 M) and the resulting mixture was heated to 50 C for 1 hr then cooled to -5 C (internal temperature). A solution of NaNO2 (1.77 g, 25.7 mmol, 1.07 eq) dissolved in water (7 mL) was added to the reaction mixture. During addition, the internal temperature was kept below 0 C. After addition the mixture was stirred for additional 10 min at -5 C. The aqueous thionyl chloride mixture was cooled to -5 C (internal temperature) and CuCl (24 mg, 0.24 mmol, 0.01 eq) was added. The freshly formed diazonium mixture was added slowly to the aqueous thionyl chloride mixture while keeping the internal temperature below 0 C. After addition, the mixture was stirred at -5 C for additional 20 min. The resulting precipitate was filtered and dissolved in THF (100 mL). The sulfonyl chloride in THF was added slowly to a stirring mixture of NH4OH (82 mL) at -10 C (internal temperature). During the addition, the internal temperature was kept below -5 C. After the addition, the mixture was poured into 2 M HCl (600 mL). At pH 2, the aqueous layer was extracted with EtOAc (3 x 100 mL). The combined organic layer was dried over Na2SO4 and concentrated yielding the product (5.34 g, 82%).
References:
WO2021/61803,2021,A1 Location in patent:Paragraph 0112; 0183