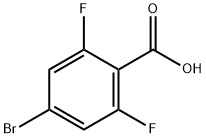
4-Bromo-2,6-difluorobenzoic acid synthesis
- Product Name:4-Bromo-2,6-difluorobenzoic acid
- CAS Number:183065-68-1
- Molecular formula:C7H3BrF2O2
- Molecular Weight:237

124-38-9
122 suppliers
$214.00/14L

461-96-1
477 suppliers
$6.00/10g

183065-68-1
268 suppliers
$8.00/5g
Yield:183065-68-1 84%
Reaction Conditions:
Stage #1: 3,5-difluorobromobenzenewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -78; for 1.75 h;
Stage #2: carbon dioxide at 20; for 16 h;
Steps:
69.A Step A: 4-bromo-2,6-difluoro-benzoic acid
70 mL of THF was cooled to -78°C, and diisopropylamine (4 mL, 28.5 mmol) and butyllithium (13 mL, 27.2mmol, 2.1 M hexane solution) were sequentially added thereto. The mixture was stirred at -78°C for 1 hour, and 1-bromo-3,5-difluorobenzene (5.0 g, 25.9 mmol) dissolved in 15 mL was slowly added thereto. The mixture was stirred at -78°Cfor 45 minutes, and the reaction solution was transferred to a beaker containing solid carbon dioxide and stirred at roomtemperature for 16 hours. The reaction solution was adjusted to pH 3 by the addition of 1N HCl aqueous solution andextracted with EtOAc. The organic layer was collected and dried with MgSO4 to obtain the title compound (5.15 g, 84 %).1H-NMR (CDCl3) δ 7.20 (2H, m)
References:
EP3239143,2017,A2 Location in patent:Paragraph 0181

461-96-1
477 suppliers
$6.00/10g

183065-68-1
268 suppliers
$8.00/5g
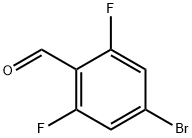
537013-51-7
224 suppliers
$10.00/1g

183065-68-1
268 suppliers
$8.00/5g
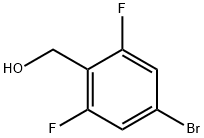
162744-59-4
171 suppliers
$8.00/1g

183065-68-1
268 suppliers
$8.00/5g
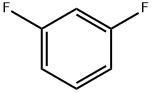
372-18-9
413 suppliers
$9.00/1g

183065-68-1
268 suppliers
$8.00/5g