
4-broMo-2-chloro-5-(trifluoroMethoxy)aniline synthesis
- Product Name:4-broMo-2-chloro-5-(trifluoroMethoxy)aniline
- CAS Number:115844-00-3
- Molecular formula:C7H4BrClF3NO
- Molecular Weight:290.46
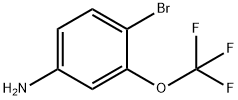
116369-25-6
72 suppliers
$108.00/250mg

115844-00-3
22 suppliers
inquiry
Yield:115844-00-3 64%
Reaction Conditions:
with N-chloro-succinimide in quinoclamine;ethyl acetate;
Steps:
62.a (a)
(a) 4-Bromo-2-chloro-5-trifluoromethoxy-phenylamine To a mixture of 4-bromo-3-trifluoromethoxy-phenylamine (2.0 g, 7.8 mmol) in ACN (60 mL) was slowly added a solution of N-chlorosuccinimide (1.0 g, 7.8 mmol) in ACN (40 mL). The reaction mixture was heated at 60° C. overnight and extracted with ethyl actetate/water. The organic layer was dried over sodium sulfate and purified by flash chromatography (40 g column, 100% hexanes to 10% EtOAc:hexanes) to produce the desired product as an orange-ish colored oil (1.4 g, 64% yield).
References:
US2012/114600,2012,A1