
4-BROMO-2-FLUOROBENZYLMETHYLSULFONE synthesis
- Product Name:4-BROMO-2-FLUOROBENZYLMETHYLSULFONE
- CAS Number:886498-34-6
- Molecular formula:C8H8BrFO2S
- Molecular Weight:267.12
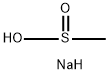
20277-69-4
267 suppliers
$10.00/1g
76283-09-5
255 suppliers
$9.00/1g

886498-34-6
23 suppliers
$110.00/250mg
Yield:886498-34-6 92 %
Reaction Conditions:
in ethanol at 80;
Steps:
1.2.1 4-Bromo-2-fluoro-1-((methylsulfonyl)methyl)benzene
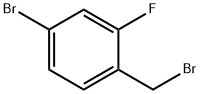
(S3) [00163] Degassed EtOH (22 mL) was added to a mixture of 4-bromo-1-(bromomethyl)-2-fluorobenzene (2.95 g, 11.0 mmol) and sodium methanesulfinate (1.46 g, 14.3 mmol, 1.3 eq) at rt. The resultant reaction mixture stirred at 80 °C for 1 h before being concentrated in vacuo. The residue was partitioned between EtOAc and H2O and the organic layer was washed additionally with H2O ( 3), brine, dried over MgSO4 and concentrated in vacuo. The crude mixture was washed on a P3 frit (eluent hexane/ Et2O, 1:1) and concentrated in vacuo to give the desired product S3 in 92% yield as a colorless solid (2.71 g). 1H NMR (300 MHz, CDCl3) δ ppm 7.42 - 7.33 (3H, m, Ar-H), 4.27 (2H, s, CH2SO2CH3), 2.83 (3H, br q, J = 0.9 Hz, CH3); LRMS [M-SO2Me]+ 186.9.
References:
WO2022/248887,2022,A1 Location in patent:Paragraph 00163