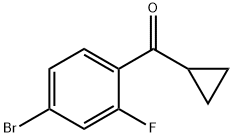
4-BROMO-2-FLUOROPHENYL CYCLOPROPYL KETONE synthesis
- Product Name:4-BROMO-2-FLUOROPHENYL CYCLOPROPYL KETONE
- CAS Number:898790-15-3
- Molecular formula:C10H8BrFO
- Molecular Weight:243.07
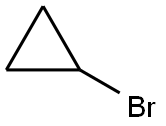
4333-56-6
377 suppliers
$6.00/5g
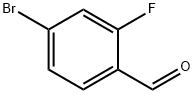
57848-46-1
382 suppliers
$9.00/1g

898790-15-3
22 suppliers
$75.00/250mg
Yield:898790-15-3 2.19 g
Reaction Conditions:
Stage #1: cyclopropyl bromidewith magnesium in tetrahydrofuran at 20; for 1 h;
Stage #2: 4-bromo-2-fluorobenzaldehyde in tetrahydrofuran at 20; for 48 h;
Steps:
148.1 (1) Synthesis of (4-bromo-2-fluorophenyl)cyclopropyl methanone [148-1] (hereinafter referred to as a compound [148-1])
To magnesium (265 mg), tetrahydrofuran (2 mL) was added, and cyclopropyl bromide (1.1 mL) was added slowly at room temperature, and then the reaction mixture was stirred at room temperature for 1 hour. To a solution of 4-bromo-2-fluorobenzaldehyde (2.0 g) in tetrahydrofuran (20 mL) was added the solution of the obtained Grignard reagent in tetrahydrofuran at 0°C, and then the reaction mixture was stirred at room temperature for 2 days. To the reaction mixture was added a saturated aqueous solution of ammonium chloride, and the reaction mixture was extracted with ethyl acetate. The obtained organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography to give the titled compound (2.19 g) as a brown oil. 1H-NMR (400 MHz, CDCl3) δ: 7.70-7.35 (1H, m), 7.40-7.20 (2H, m), 2.60-2.50 (1H, m), 1.43-1.25 (2H, m), 1.11-1.05 (2H, m).
References:
EP2669270,2013,A1 Location in patent:Paragraph 0860-0861

57848-46-1
382 suppliers
$9.00/1g

898790-15-3
22 suppliers
$75.00/250mg