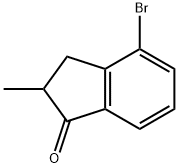
4-Bromo-2-methyl-1-indanone synthesis
- Product Name:4-Bromo-2-methyl-1-indanone
- CAS Number:174702-59-1
- Molecular formula:C10H9BrO
- Molecular Weight:225.08
869063-66-1
0 suppliers
inquiry

174702-59-1
87 suppliers
$45.00/1 g
Yield: 125.5 g (75%)
Reaction Conditions:
with thionyl chloride;AlCl3 in dichloromethane
Steps:
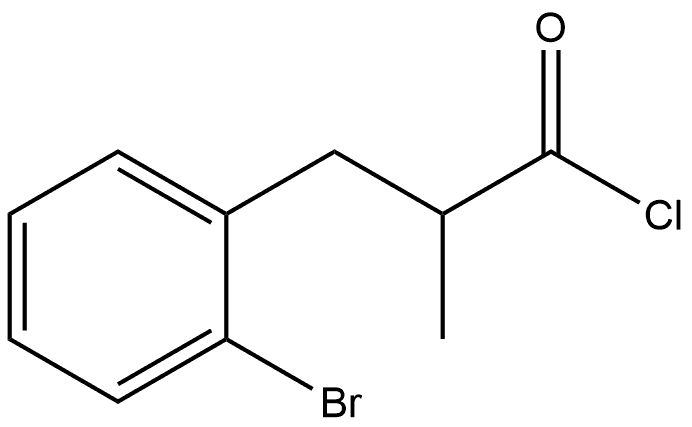
1 4-bromo-2-methyl-1-indanone via 3-(2-bromophenyl)-2-methylpropionyl chloride via 3-(2-bromophenyl)-2-methylpropionic acid
A mixture of this acid and 160 ml of SOCl2 was stirred for 24 hours at ambient temperature. Thionyl chloride was distilled off. The crude 3-(2-bromophenyl)-2-methylpropionyl chloride dissolved in 270 ml of CH2Cl2 was added dropwise with vigorous stirring to a suspension of 136 g (1.02 mol) of AlCl3 in 1350 ml of CH2Cl2 for 1 hour at 0° C. Then, this mixture was refluxed for 3 hours, cooled to ambient temperature, and poured on 500 cm3 of ice. The organic layer was separated. The aqueous layer was extracted with 3*300 ml of methyl-tert-butyl ether. The combined extract was dried over K2CO3 and evaporated to dryness. Fractional distillation gave 4-bromo-2-methyl-1-indanone, b.p. 131-134° C./2 mm Hg. Yield, 125.5 g (75%) of a colorless solid. Anal. calc. for C10H9BrO: C, 53.36; H, 4.03. Found: C, 53.19; H, 3.98. 1H NMR (300 MHz, CDCl3): δ 7.76 (d, J=7.6 Hz, 1H, 7-H), 7.71 (d, J=7.6 Hz, 1H, 5-H), 7.28 (t, J=7.6 Hz, 1H, 6-H), 3.36 (dd, J=17.5 Hz, J=7.6 Hz, 1H, 3-H), 2.70-2.82 (m, 1H, 2-H), 2.67 (dd, J=17.5 Hz, J=3.8 Hz, 1H, 3'-H), 1.34 (d, J=7.3 Hz, 3H, 2-Me). 13C NMR (75 MHz, CDCl3): δ 208.3, 152.9, 138.2, 137.2, 129.0, 122.6, 122.0, 41.8, 35.7, 16.0.
References:
Voskoboynikov, Alexander Z.;Izmer, Vyatcheslav V.;Asachenko, Andrey F.;Nikulin, Mikhail V.;Ryabov, Alexey N.;Lebedev, Artyom Y.;Coker, Catalina L.;Canich, Jo Ann M. US2007/135597, 2007, A1
19829-31-3
161 suppliers
$16.00/1g

174702-59-1
87 suppliers
$45.00/1 g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

174702-59-1
87 suppliers
$45.00/1 g
6630-33-7
408 suppliers
$9.00/10g

174702-59-1
87 suppliers
$45.00/1 g