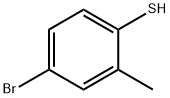
4-Bromo-2-methylbenzenethiol synthesis
- Product Name:4-Bromo-2-methylbenzenethiol
- CAS Number:14395-51-8
- Molecular formula:C7H7BrS
- Molecular Weight:203.1

1360628-93-8
0 suppliers
inquiry

14395-51-8
9 suppliers
$1300.00/5g
Yield:14395-51-8 8.2 g
Reaction Conditions:
with sodium hydroxide in water;isopropyl alcohol at 80; for 24 h;
Steps:
6
A mixture of 10 g of 1B, 50 ml of an aqueous 20% sodium hydroxide solution, and 50 ml of isopropanol was stirred at 80° C. for 24 hours. To the reaction mixture, 10% hydrochloric acid was added and the mixture was extracted with chloroform. The organic layer was washed with water, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure. The residue thus obtained was subjected to silica gel column chromatography to obtain 8.2 g of 1A. (0168) 1H-NMR (CDCl3) δ: 7.30 (1H, d, J=2.1 Hz), 7.19 (1H, dd, J=8.2, 2.1 Hz), 7.13 (1H, d, J=8.2 Hz), 3.28 (1H, s), 2.30 (3H, s).
References:
US2017/107196,2017,A1 Location in patent:Paragraph 0167; 0168
18619-15-3
18 suppliers
$28.60/25MG

14395-51-8
9 suppliers
$1300.00/5g

2362-12-1
183 suppliers
$5.00/1g

14395-51-8
9 suppliers
$1300.00/5g
685126-81-2
0 suppliers
inquiry

14395-51-8
9 suppliers
$1300.00/5g
42943-68-0
9 suppliers
inquiry

14395-51-8
9 suppliers
$1300.00/5g