
4-BROMO-2-PIPERIDINOBENZENECARBALDEHYDE synthesis
- Product Name:4-BROMO-2-PIPERIDINOBENZENECARBALDEHYDE
- CAS Number:643094-36-4
- Molecular formula:C12H14BrNO
- Molecular Weight:268.15
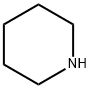
110-89-4
0 suppliers
$15.96/100ML
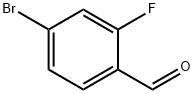
57848-46-1
382 suppliers
$9.00/1g

643094-36-4
18 suppliers
$89.00/1g
Yield:643094-36-4 92%
Reaction Conditions:
in N,N-dimethyl acetamide at 120; for 3 h;
Steps:
56.1 Step 1: Preparation of 3-bromo-5-(piperidin-l-yl)benzald hyde
A 20 mL screwtop vial was charged with 4-bromo-2-fluorobenzaldehyde (200 mg, 0.985 mmol), piperadine (1 16 μ, 1.19 mmol), and DMA (1 mL). The reaction was heated to 120 °C for 3h. The reaction was diluted in EtOAc and extracted with brine (3X). The organics were dried (Na2S04), filtered, and concentrated under reduced pressure. The residue was chromatographed on a silica gel column (0% to 20% EtOAc in hexanes) and yielded 4-bromo-2-(pyrrolidin-l-yl)benzaldehyde (242 g, 92%). XH NMR 400 MHz (CDC13) δ10.21 (s, 1H), 7.66 (dd, J= 8.3, 2.4 Hz, 1H), 7.31 - 7.17 (m, 2H), 4 (m, 4H), 1.78 (m, 4H), 1.71 - 1.60 (m, 2H). LCMS (ESI, m/z): 268 [M+H]+.
References:
WO2013/103973,2013,A1 Location in patent:Paragraph 00219