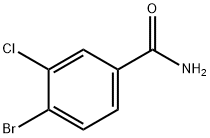
4-BroMo-3-chlorobenzaMide synthesis
- Product Name:4-BroMo-3-chlorobenzaMide
- CAS Number:1228826-41-2
- Molecular formula:C7H5BrClNO
- Molecular Weight:234.48
25118-59-6
190 suppliers
$6.00/1g

1228826-41-2
23 suppliers
$45.00/25mg
Yield:1228826-41-2 100%
Reaction Conditions:
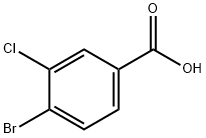
Stage #1: 4-bromo-3-chlorobenzoic acidwith thionyl chloride in toluene; for 20.5 h;Reflux;
Stage #2: with ammonia in toluene at 20; for 1.16667 h;
Steps:
23.1
To a suspension of 4-bromo-3-chlorobenzoic acid (2.00 g, 8.49 mmol) in toluene (10 mL) at ambient temperature was added thionyl chloride (1.85 mL, 25.4 mmol). The reaction mixture was heated at reflux for 20.5 hours, then distilled for 1 hour, collecting 9 mL of distillate. Toluene (10 mL) was added and the reaction was distilled for 30 minutes, collecting 10 mL of distillate. The reaction mixture was cooled to ambient temperature resulting in a suspension. Toluene (10 mL) saturated with ammonia gas was added to the reaction mixture followed by bubbling ammonia gas through the reaction for 10 minutes. The reaction was stirred at ambient temperature for 1 hour. Heptane (10 mL) was added, and the reaction stirred at ambient temperature for 15 minutes, then 0 °C for 1 hour. The solids were collected by vacuum filtration, rinsed with excess heptane, and dried to afford 4-bromo-3- chlorobenzamide (2.327 g, 116.8%) as a white solid which was carried on without further purification.
References:
WO2011/29027,2011,A1 Location in patent:Page/Page column 72; 73