4-Bromo-3-fluoro-4-methylbenzhydrol synthesis
- Product Name:4-Bromo-3-fluoro-4-methylbenzhydrol
- CAS Number:853348-88-6
- Molecular formula:C14H12BrFO
- Molecular Weight:295.15
Yield:853348-88-6 65%
Reaction Conditions:
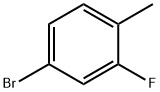
Stage #1: 2-fluoro-4-bromotoluenewith magnesium;iodine in tetrahydrofuran; for 1.66667 h;Heating / reflux;
Stage #2: 4-bromo-benzaldehyde in tetrahydrofuran at 0 - 20; for 2.5 h;
Steps:
1
Dried magnesium turnings (4.24 g, 0.55 mole) were stirred with a heavy stirrer bar under argon for 16 hours. A few crystals of iodine were added followed by the addition a solution of 4-bromo-2-fluorotoluene (94.5 g, 0.5 mole) in tetrahydrofuran [THF] (200ml). This addition took place over about 40 minutes and the solution was allowed to reflux during the addition. The resulting solution was stirred for 1 hour.4-Bromobenzaldehyde (71.7 g 0.39 mole) in THF (200 ml) was cooled to0 C then treated with the above Grignard solution. The addition
References:
WO2005/51399,2005,A1 Location in patent:Page/Page column 21-22
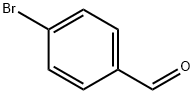
1122-91-4
672 suppliers
$9.00/10g
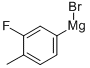
185077-02-5
30 suppliers
$226.00/50mL

853348-88-6
4 suppliers
inquiry