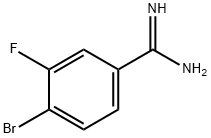
4-BROMO-3-FLUORO-BENZAMIDINE synthesis
- Product Name:4-BROMO-3-FLUORO-BENZAMIDINE
- CAS Number:780721-73-5
- Molecular formula:C7H6BrFN2
- Molecular Weight:217.04

133059-44-6
229 suppliers
$5.00/1g

780721-73-5
6 suppliers
inquiry
Yield:780721-73-5 60%
Reaction Conditions:
Stage #1: 4-bromo-3-fluorobenzonitrilewith hydrogenchloride in ethanol;chloroform at 20;Cooling;
Stage #2: with ammonium carbonate in ethanol at 20;
Steps:
5.7. 4-Chlorobenzamidine hydrochloride (12b)
General procedure: Hydrogen chloride gas was passed through a solution of 4-chlorobenzonitrile (9b, 25.0 g) in chloroform (350 mL) and ethanol (100 mL) at -78 °C for 0.5 h. Then the solution was warmed up to room temperature, and stirred at room temperature overnight. The solution was evaporated in vacuo, and the resulting residue was dissolved with ethanol (500 mL). To the solution was added ammonium carbonate (90.0 g), and the reaction mixture was stirred at room temperature for 3 days. To the mixture was added water (300 mL), and ethanol was removed by concentration in vacuo. The resulting solid was collected by filtration, washed with water and dried in vacuo to give 12b (25.4 g, 71%) as a white solid.
References:
Negoro, Kenji;Yonetoku, Yasuhiro;Misawa-Mukai, Hana;Hamaguchi, Wataru;Maruyama, Tatsuya;Yoshida, Shigeru;Takeuchi, Makoto;Ohta, Mitsuaki [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 17,p. 5235 - 5246]