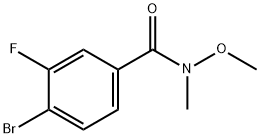
4-BROMO-3-FLUORO-N-METHOXY-N-METHYL-BENZAMIDE synthesis
- Product Name:4-BROMO-3-FLUORO-N-METHOXY-N-METHYL-BENZAMIDE
- CAS Number:343564-56-7
- Molecular formula:C9H9BrFNO2
- Molecular Weight:262.08

6638-79-5
554 suppliers
$6.00/25g

343564-56-7
22 suppliers
inquiry
Yield:343564-56-7 99%
Reaction Conditions:
Stage #1: 4-bromo-3-fluorobenzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 2 h;
Stage #2: N,O-dimethylhydroxylamine*hydrochloridewith potassium carbonate in dichloromethane;water at 20; for 2 h;
Steps:
74.1
Oxalyl chloride (38.1 mL, 0.450 mol) was slowly added to a mixture of 4-bromo-3-fluorobenzoic acid (49.3 g, 0.225 mol) in methylene chloride (300 mL), then DMF (1.0 mL) was added and the reaction was stirred at RT for 2 h. The volatiles were removed under reduced pressure and co-evaporated with toluene for 3 times. The residue was then dissolved in methylene chloride (100 mL). The solution was added dropwise to a mixture of N,O-dimethyl-hydroxylamine hydrochloride (30.7 g, 0.315 mol) and potassium carbonate (120 g, 0.90 mol) in methylene chloride (300 mL) and water (300 mL). The reaction was stirred at RT for 2 h. The organic layer was separated. The aqueous layer was extracted with methylene chloride (2*50 mL). The combined organic layers were washed with brine, dried over MgSO4, filtered, and concentrated under reduced pressure to give the product (58.5 g, 99%). Analytical LCMS: (M+H)+=261.9/263.9.
References:
US2008/39457,2008,A1 Location in patent:Page/Page column 40-41

6638-79-5
554 suppliers
$6.00/25g
153556-42-4
253 suppliers
$10.00/1g

343564-56-7
22 suppliers
inquiry

695188-21-7
33 suppliers
$116.00/1g

6638-79-5
554 suppliers
$6.00/25g

343564-56-7
22 suppliers
inquiry

153556-42-4
253 suppliers
$10.00/1g

343564-56-7
22 suppliers
inquiry
