
4-Bromo-3-hydroxyquinoline synthesis
- Product Name:4-Bromo-3-hydroxyquinoline
- CAS Number:32435-61-3
- Molecular formula:C9H6BrNO
- Molecular Weight:224.05
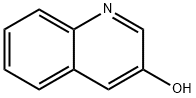
580-18-7
157 suppliers
$6.00/250mg

32435-61-3
24 suppliers
$330.00/100mg
Yield:32435-61-3 78%
Reaction Conditions:
with bromine;acetic acid at 60; for 0.0833333 - 0.166667 h;
Steps:
Synthesis of 4-bromo-3-hydroxy quinoline (III)
In15 mL of glacial acetic acid, 3-hydroxy quinoline (II)(0.01 mol) was dissolved, then 10 mL of bromine (0.01mol) in glacial acetic acid was added dropwise to compound(II) solution with stirring at 60°C. After 5-10 min the bromine color was discharged and a yellowsolution remained. At this point, 0.5-1.0 mL of thebromine-AcOH solution was added with stirring atroom temperature for 30 min. The reaction mixturewas poured into ice-cooled water with stirring, and theresulting product was filtered off, washed with water,and dried. Finally, the product was crystallized fromethanol to give 4-bromo-3-hydroxy quinoline (III).Colorless crystals, yield 78%, mp 180-182°C(reported: 182-183°C) [30]. IR (KBr) max: 3385(NH), 1642 (C=N), 1611, 1593 (C=C), 1083 (C-O)cm-1. 1H NMR (400 MHz, DMSO-d6) : 7.62 (d, J =8.7 Hz, 1H, quinoline CH), 7.94 (t, J = 2.1 Hz, 1H,quinoline CH), 7.96 (t, J = 2.1 Hz, 1H, quinolineCH), 8.14 (s, 1H, quinoline CH), 8.18 (d, J = 2.1 Hz,1H, quinoline CH), 12.46 (s, 1H, OH) ppm. 13CNMR (101 MHz, DMSO-d6) : 119.23 (C4 quinoline),124.26 (C6 quinoline), 127.96 (C4', 5 quinoline),129.708 (C8 quinoline), 137.194 (C7 quinoline),146.059 (C2 quinoline), 147.784 (C8' quinoline),159.638 (C3 quinoline) ppm. MS: m/z (%) = 225 (M+ +2, 90.00), 223 (M+, 100). Anal. calcd for C9H6BrNO(223.85): C, 48.25; H, 2.70; N, 6.25. Found: C, 48.08;H, 2.78; N, 6.41.
References:
Amal, M. Imam;Islam, Zaki [Russian Journal of Bioorganic Chemistry,2020,vol. 46,# 6,p. 1099 - 1109][Bioorg. Khim.]
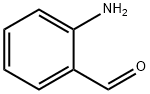
529-23-7
244 suppliers
$26.00/1g

32435-61-3
24 suppliers
$330.00/100mg