
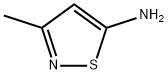
4-BROMO-3-METHYL-ISOTHIAZOL-5-YLAMINE synthesis
- Product Name:4-BROMO-3-METHYL-ISOTHIAZOL-5-YLAMINE
- CAS Number:85508-99-2
- Molecular formula:C4H5BrN2S
- Molecular Weight:193.06
24340-76-9
51 suppliers
$192.00/1g

85508-99-2
40 suppliers
$110.00/50mg
Yield:85508-99-2 88%
Reaction Conditions:
with N-Bromosuccinimide in tetrachloromethane at 20 - 65; for 1.66667 h;
Steps:
112
5-Amino-3-methyl isothiazole hydrochloride (5.00 g, 33.2 mmol) was added to water (35 mL). The insolubles were filtered and the filtrate's pH was adjusted to 10 with the addition of 2N NaOH. The mixture was stirred for five minutes and extracted with ethyl ether. The organic layer was separated and the aqueous layer was saturated with NaCl, extracted with ethyl ether (100 mL, 2×). The combined ether extracts were washed with brine, dried over sodium sulfate and then concentrated to afford compound 112 as dark orange oil, 3.12 g (82%). 1H-NMR (400 MHz, DMSO-d6 6 6.5 (bs, 2H), 5.9 (s, 1H), 2.1 (s, 3H). 5-amino-3-methyl isothiazole (1.00 g, 8.75 mmol) was slurried in CCl4 (30 mL) under an atmosphere of argon. N-Bromosuccinimide (1.56 g, 8.75 mmol) was added portion-wise to the amine slurry over a 10 minute period at room temperature. The reaction stirred at 65° C. for 1.5 hours. Thin layer chromatography (DCM/Hexanes 1:1) indicates the reaction is complete. The reaction mixture was cooled to room temperature and diluted with ethyl ether (40 mL). The resulting mixture was cooled to 5° C. for 30 minutes and filtered to remove any solid material. The filtrate was concentrated to yield a dark red solid that was dissolved in ethyl acetate and washed with water (100 mL, 2×). The organic layer was separated, washed with brine, dried over anhydrous sodium sulfate, and concentrated under vacuum to afford compound 112 as a dark red solid (1.49 g, 88%). This was used without further purification. 1H-NMR (400 MHz, DMSO-d6) δ 6.7 (bs, 2H), 2.2 (s, 3H).
References:
US2007/105864,2007,A1 Location in patent:Page/Page column 150-151