
4-BROMO-5,6,7,8-TETRAHYDROISOQUINOLIN-8-AMINE synthesis
- Product Name:4-BROMO-5,6,7,8-TETRAHYDROISOQUINOLIN-8-AMINE
- CAS Number:1428651-87-9
- Molecular formula:C9H11BrN2
- Molecular Weight:227.1
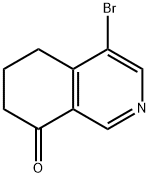
1428651-86-8
37 suppliers
inquiry

1428651-87-9
8 suppliers
inquiry
Yield:1428651-87-9 85%
Reaction Conditions:
Stage #1: 4-bromo-6,7-dihydroisoquinolin-8(5H)-onewith methanol;ammonia;titanium(IV)isopropoxide at 20; for 5 h;
Stage #2: with sodium tetrahydroborate at 0 - 20; for 2 h;
Steps:
A-2; D [D] (rac)-4-Bromo-5,6,7,8-tetrahydroisoquinolin-8-amine
[D] (rac)-4-Bromo-5,6,7,8-tetrahydroisoquinolin-8-amine[0351]4-Bromo-6,7-dihydroisoquinolin-8(5H)-one (4.81 g, 21.3 mmol), titanium (IV) isopropoxide (12.5 mL, 42.6 mmol) and ammonia, 2.0 M solution in MeOH (53.2 mL, 106 mmol) were stirred at RT for 5 h. The reaction was cooled to 0° C. and NaBH4 (1.21 g, 31.9 mmol) was added portionwise over 10 min; the resulting mixture was stirred at RT for an additional 2 h. The reaction was quenched by pouring it into aq. ammonium hydroxide (25%), the precipitate was filtered and washed with EtOAc (3×, each time suspended in AcOEt and stirred for 5 min). The organic layer was separated and the remaining aqueous layer was extracted with EtOAc. The combined organic extracts were extracted with 1 M HCl. The acidic aqueous extracts were washed with ethyl acetate (1×), then treated with aqueous sodium hydroxide (2 M) to give pH 10-12, and extracted with EtOAc (3×). The combined organic extracts were washed with brine, dried (Na2SO4), and concentrated in vacuo to afford the title compound (4.11 g, 85%) as brown solid. MS: 225 (M+-H, 1Br).
References:
US2013/79365,2013,A1 Location in patent:Paragraph 0351; 0352
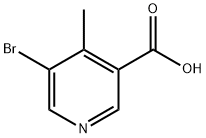
677702-58-8
62 suppliers
$65.00/100mg

1428651-87-9
8 suppliers
inquiry
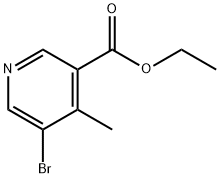
1428651-84-6
20 suppliers
inquiry

1428651-87-9
8 suppliers
inquiry

1428651-85-7
5 suppliers
inquiry

1428651-87-9
8 suppliers
inquiry