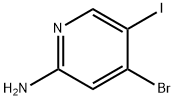
4-broMo-5-iodopyridin-2-aMine synthesis
- Product Name:4-broMo-5-iodopyridin-2-aMine
- CAS Number:1186115-39-8
- Molecular formula:C5H4BrIN2
- Molecular Weight:298.91
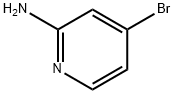
84249-14-9
363 suppliers
$6.00/1g

1186115-39-8
53 suppliers
$46.00/100mg
Yield: 35%
Reaction Conditions:
with N-iodo-succinimide in chloroform;N,N-dimethyl-formamide at 60; for 10 h;
Steps:
2.1 Step 1: Preparation of 4-bromo-5-iodo-pyridin-2-ylamine
Dissolve 2-amino-4-bromopyridine (1.0 g, 5.8 mmol) in a mixed solvent of N, N-dimethylformamide (6.0 mL) and chloroform (20.0 mL), and add N-iodosuccinimide Amine (2.6 g, 11.6 mmol) was reacted at 60 ° C for 10 hours.After the reaction is completed, cool to room temperature and pour the reaction solution into water.Extracted with ethyl acetate, combined organic phases, dried, filtered, concentrated,The residue was purified by silica gel column chromatography (eluent: ethyl acetate / petroleum ether = 1/2),The title compound (0.6 g, yield: 35%) was obtained in this step.
References:
Sichuan Kelun Botai Bio-pharmaceutical Co., Ltd.;Liu Jinming;Cai Jiaqiang;He Ting;Wang Lichun;Wang Jingyi CN110240593, 2019, A Location in patent:Paragraph 0164-0168