
4-BROMO-6-FLUORO (1H)INDAZOLE synthesis
- Product Name:4-BROMO-6-FLUORO (1H)INDAZOLE
- CAS Number:885520-70-7
- Molecular formula:C8H5BrFN
- Molecular Weight:214.03
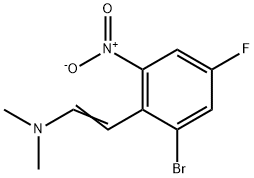
1354219-92-3
0 suppliers
inquiry

885520-70-7
110 suppliers
$37.00/100mg
Yield:885520-70-7 800 mg
Reaction Conditions:
with iron;acetic acid at 110; for 1.5 h;
Steps:
110.3 3rd step
An acetic acid (20 ml) solution containing the deep brown oily matter obtained in the 2nd step was added to amixture of iron powder (3.61 g) and acetic acid (20 ml) at 110°C for 30 minutes. The resulting mixture was stirred for 1hour and then diluted with ethyl acetate. Insoluble matter was removed by filtration with Celite, the filtrate was washedwith water and 1M hydrochloric acid (x3). The obtained organic layer was poured into a saturated aqueous sodiumhydrogen carbonate solution to separate the organic layer, and the organic layer was washed with water and saturated saline and dried over anhydrous sodium sulfate. Thereafter, activated carbon was added and insoluble matter wasremoved by filtration with Celite. The solvent was distilled away under reduced pressure, and light brown oily matter of4-bromo-6-fluoro-1H-indole (880 mg) was thus obtained.MS (ESI m/z): 214, 216 (M+H)RT (min): 1.561H-NMR (DMSO-d6, 300MHz) δ:11.53 (br, 1H), 7.46 (t, 1H, J = 3.0Hz), 7.24 (dd, 1H, J = 5.6, 3.0 Hz), 7.19 (dd, 1H, J =9.2, 2.0Hz), 6.39 (d, 1H, J = 2.0Hz)
References:
EP2589592,2018,B1 Location in patent:Paragraph 0755; 0758
![Pyrrolidine, 1-[2-(2-bromo-4-fluoro-6-nitrophenyl)-1-methylethenyl]-](/CAS/20200611/GIF/1093066-65-9.gif)
1093066-65-9
0 suppliers
inquiry

885520-70-7
110 suppliers
$37.00/100mg
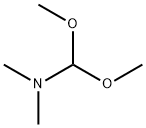
4637-24-5
592 suppliers
$5.00/25g
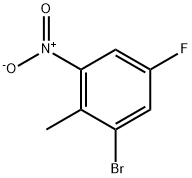
502496-33-5
153 suppliers
inquiry

885520-70-7
110 suppliers
$37.00/100mg

1181566-93-7
1 suppliers
inquiry

885520-70-7
110 suppliers
$37.00/100mg

502496-33-5
153 suppliers
inquiry

885520-70-7
110 suppliers
$37.00/100mg