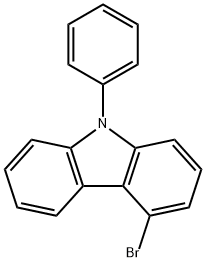
4-broMo-9-phenyl-9H-carbazole synthesis
- Product Name:4-broMo-9-phenyl-9H-carbazole
- CAS Number:1097884-37-1
- Molecular formula:C18H12BrN
- Molecular Weight:322.2
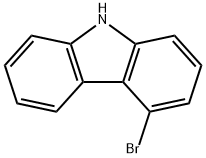
3652-89-9
214 suppliers
$9.00/100mg
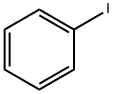
591-50-4
470 suppliers
$10.00/1g

1097884-37-1
79 suppliers
$16.00/100mg
Yield:1097884-37-1 97%
Reaction Conditions:
with copper (I) iodide;potassium carbonate;1,3-bis(pyridin-2-yl)propane-1,3-dione in N,N-dimethyl-formamide at 110;Inert atmosphere;
Steps:
Preparation of 4-bromo-9-phenyl-9H-carbazole 3a
A 500 ml four-neck flask is initially charged with 15.0 g (61.0 mmol, 1.0 eq) of 4-bromo-9H-carbazole 1a (CAS 3652-89-9) together with 13.6 ml (122 mmol, 2.0 eq) of iodobenzene 2a and 16.8 g (122 mmol, 2.0 eq) of potassium carbonate, which are dissolved in 180 ml of dried DMF. After degassing by means of a nitrogen stream for 30 minutes, 1.38 g (6.10 mmol, 0.10 eq) of 1,3-di(2-pyridyl)-1,3-propanedione and 1.16 g (6.10 mmol, 0.10 eq) of copper(I) iodide are added. The mixture is stirred at 110° C. overnight and, after the reaction has ended, the solvent is removed on a rotary evaporator. The residue is taken up in 250 ml of DCM, conc. ammonium chloride solution is added and the mixture is filtered through Celite. Subsequently, the phases are separated, the aqueous phase is extracted twice with 100 ml each time of DCM and the combined organic phases are finally washed with water. After drying over sodium sulphate and removing the solvent under reduced pressure, the oily residue together with heptane is filtered through silica gel and the solvent is again removed on a rotary evaporator. 19.0 g(59.0 mmol, 97%) of a colourless oil 3a are obtained.
References:
US10957859,2021,B2 Location in patent:Page/Page column 201

108-86-1
498 suppliers
$10.00/5g

3652-89-9
214 suppliers
$9.00/100mg

1097884-37-1
79 suppliers
$16.00/100mg

3652-89-9
214 suppliers
$9.00/100mg
100-63-0
359 suppliers
$10.00/1g

1097884-37-1
79 suppliers
$16.00/100mg
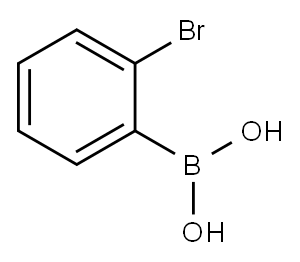
244205-40-1
322 suppliers
$9.00/1g

1097884-37-1
79 suppliers
$16.00/100mg
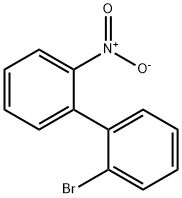
17613-47-7
12 suppliers
inquiry

1097884-37-1
79 suppliers
$16.00/100mg