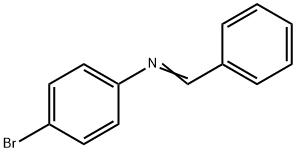
4-Bromo-N-benzylideneaniline synthesis
- Product Name:4-Bromo-N-benzylideneaniline
- CAS Number:780-20-1
- Molecular formula:C13H10BrN
- Molecular Weight:260.13

100-52-7
944 suppliers
$20.37/250g

106-40-1
458 suppliers
$12.00/5g

780-20-1
1 suppliers
$28.60/50MG
Yield:780-20-1 98%
Reaction Conditions:
with PEG-400 in neat (no solvent) at 20; for 0.75 h;Green chemistry;
Steps:
Preparation of Schiff base and their derivatives
General procedure: Initially, benzaldehyde (1.0 mmol) is added to PEG-400 (10 μL), and then aniline (1.0 mmol) is added to the reaction. The reaction mixture was agitated for 45 min at room temperature using a magnetic stirrer. After that, the product is monitored by TLC. The desired outcome is crystallized by hexane and ethyl acetate into ice. The product's solid form, i.e., Schiff base (N-benzylideaniline), is filtered and stored for characterization.
References:
Jindal;Kumar;Sharma;Chauhan [Oriental Journal of Chemistry,2022,vol. 38,# 4,p. 998 - 1002]

100-52-7
944 suppliers
$20.37/250g
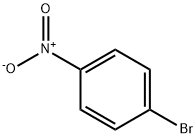
586-78-7
9 suppliers
$14.00/5g
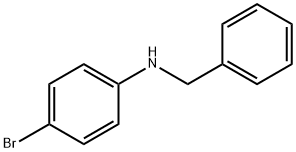
2879-83-6
28 suppliers
$112.00/100mg

780-20-1
1 suppliers
$28.60/50MG

106-40-1
458 suppliers
$12.00/5g

100-51-6
1379 suppliers
$5.00/100g

780-20-1
1 suppliers
$28.60/50MG
