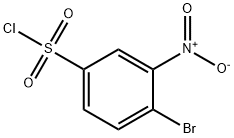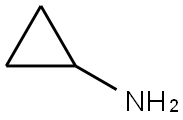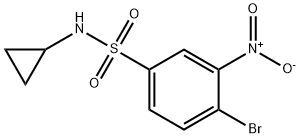
4-broMo-N-cyclopropyl-3-nitrobenzenesulfonaMide synthesis
- Product Name:4-broMo-N-cyclopropyl-3-nitrobenzenesulfonaMide
- CAS Number:1449412-80-9
- Molecular formula:C9H9BrN2O4S
- Molecular Weight:321.15
Yield:1449412-80-9 56.9%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 20;
Steps:
Compound 2 was dissolved in 100 mL ofdichloromethane and treated with cyclopropylamine (1.37 g, 1.66 mL,24 mmol, 1.2 equiv.) and DIPEA (3.10 g, 3.97 mL, 24 mmol, 1.2 equiv.). Thereaction mixture was stirred overnight at room temperature. TLC was used tomonitor the progress of the reaction. After the reaction complete, the mixturewas neutralized with 6N HCl and extracted with dichloromethane (3 × 60 mL). Theorganic layer was dried over anhydrous sodium sulfate, filtered, and evaporatedto dryness. The residue was purified by flash column chromatography (silicagel, petroleum ether/ethyl acetate = 6:1) to afford intermediate 3. Yield: 56.9%; pale yellow powder;mp:97-100 °C; 1H NMR (400 MHz, CDCl3)δ 8.34 (s, 1H), 7.94 (s, 2H), 5.13 (s, 1H), 2.33 (tt, J = 6.9, 3.6 Hz, 1H), 0.69 (dd, J =5.1, 3.5 Hz, 2H), 0.66 (d, J = 4.4 Hz, 2H); 13C NMR (101 MHz,CDCl3) δ 149.9, 140.8, 136.2, 131.2, 124.6, 119.6, 24.4, 6.4;ESI-MS: m/z 321.1 [M]- (calcd. 321.1).
References:
Bu, Huagang;Jia, Lejiao;Li, Jun;Li, Zhenyu;Li, Zhiying;Shen, Chengwu;Tang, Hui;Wu, Xingkang;Zhang, Rui [Bioorganic and medicinal chemistry letters,2020]

577-19-5
26 suppliers
$14.00/5g

1449412-80-9
15 suppliers
inquiry