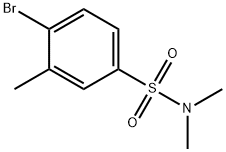
4-Bromo-N,N,3-trimethylbenzenesulphonamide synthesis
- Product Name:4-Bromo-N,N,3-trimethylbenzenesulphonamide
- CAS Number:849532-31-6
- Molecular formula:C9H12BrNO2S
- Molecular Weight:278.17

72256-93-0
131 suppliers
$8.00/1g

124-40-3
498 suppliers
$18.00/100ml

849532-31-6
22 suppliers
$90.00/250mg
Yield:849532-31-6 94%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in tetrahydrofuran at 0 - 20;
Steps:
Diisopropylethylamine (4.95 ml_, 27.8 mmol) was added at 0 °C to a stirred solution of dimethylamine (3.70 ml_, 55.6 mmol) in tetrahydrofuran (25 ml_) and the reaction mixture was stirred at room temperature for 15 minutes. A 0.1 M solution of 4- bromo-3-methyl-benzenesulfonyl chloride (3.0 g, 11 mmol) in tetrahydrofuran was added at room temperature and the reaction mixture was stirred at room temperature for 2 hours. The solvent was evaporated off under reduced pressure. The residue was diluted with ethyl acetate, and the inorganic salts were filtered through a celite bed. The filtrate was washed with 2 N aqueous HCI, dried over anhydrous sodium sulfate, filtered, and concentrated in vacuo to afford 2.90 g (94%) of 4-bromo-3- methyl-Λ/,Λ/-dimethyl-benzenesulfonamide, which was used in subsequent reactions without further purification. MS cald. for C9H12BrNO2S [M+] 278.2, obsd. 280.0.
References:
WO2010/55005,2010,A1 Location in patent:Page/Page column 84