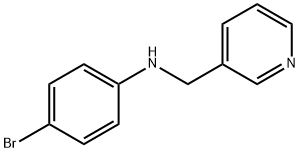
4-bromo-N-(pyridin-3-ylmethyl)aniline synthesis
- Product Name:4-bromo-N-(pyridin-3-ylmethyl)aniline
- CAS Number:84324-68-5
- Molecular formula:C12H11BrN2
- Molecular Weight:263.13
Yield:84324-68-5 94.8%
Reaction Conditions:
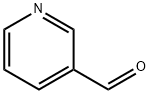
Stage #1: 3-pyridinecarboxaldehyde;4-bromo-anilinewith methanol at 85; for 3 h;
Stage #2: with sodium tetrahydroborate at 0 - 20; for 19.5 h;
Steps:
Step 1 4-bromo-N-(pyridin-3-ylmethyl)aniline
4-Bromoaniline (38.50 g, 223.8 mmcl) was added to a solution of 3- pyridinecarboxadehyde (24.00 g, 224.1 mmol) in methanol (200 mL) and thesolution heated, 85CC for ca 3hrs to give a pale yellow solution. The resulting solution was allowed to cool to RT and then cooled further with ice-water bath. Sodium borohydride (18.91 g, 500 mmol) was added portion-wise and the suspension stirred for ca 90 mins at 0C and for ca l8hrs at room temperature to give a yellow I green solution. The solution was poured into water (60 0mL) togive a pale yellow suspension. The solids were removed by filtration and washed with water (3 x 200m1). The solids were taken up in dichloromethane (500 mL) and the solution washed with water (200 mL) and saturated aqueous sodium chloride solution (200 mL). The solution wad dried over anhydrous magnesium sulphate filtered and the filtrate concentrated in vacuo to afford 4-bromo-N-(pyridin-3-ylmethyl)aniline as a pale-yellow solid (58.89 g, 94.8%). LC/MS (method B): RT = 1.10 min; m/z= 265 [M+H]. Total run time 1.90 mins.
References:
WO2015/40425,2015,A1 Location in patent:Page/Page column 65