(4-Bromo-Phenylamino)-Acetic Acid Methyl Ester synthesis
- Product Name:(4-Bromo-Phenylamino)-Acetic Acid Methyl Ester
- CAS Number:131770-56-4
- Molecular formula:C9H10BrNO2
- Molecular Weight:244.09
Yield:131770-56-4 84.34%
Reaction Conditions:
with potassium carbonate;sodium iodide in acetonitrile at 90; for 24 h;
Steps:
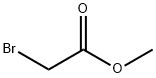
S2 Step S2, Compound 3: Preparation of methyl(4-bromophenyl)glycine:
To a solution of 4-bromoaniline (10 g, 58.13 mmol) and methyl 2-bromoacetate (9.78 g, 63.95 mmol) in acetonitrile (150 mL) was added sodium iodide (3.49 g, 23.25 mmol) and anhydrous potassium carbonate (20.09 g, 145.33 mmol),After stirring at 90°C for 24 hours, the mixture turned yellow.The 4-bromoaniline was completely reacted by liquid chromatography-mass spectrometry, and a main peak with the expected mass was detected.The reacted mixture was filtered through celite, and the organic layer was collected by distillation under reduced pressure.The residue was subjected to flash chromatography on silica gel,Purify with a gradient eluent of 0-30% ethyl acetate and petroleum ether (flash silica column, 35 mL/min),A yellow oily substance was obtained, compound 4 (11.97 g, 49.03 mmol) in 84.34% yield.
References:
CN114644836,2022,A Location in patent:Paragraph 0056-0057
51673-84-8
134 suppliers
$11.00/5g

2617-78-9
17 suppliers
$130.00/50mg

131770-56-4
6 suppliers
inquiry